Impact Factor
ISSN: 1837-9664
J Cancer 2025; 16(5):1451-1465. doi:10.7150/jca.107194 This issue Cite
Research Paper
Integrative Predictive Nomograms for Treatment Decision-Making in Resectable Synchronous Colorectal Liver Metastases
1. Department of Colorectal Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
2. Department of Pancreatic and Gastric Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
3. Department of Hepatobiliary Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
4. Department of Diagnostic Radiology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
5. Department of Plastic and Cosmetic Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
#These authors contributed equally to this work.
Received 2024-11-19; Accepted 2024-12-31; Published 2025-1-27
Abstract
Background: Currently, there is no established standard for managing resectable synchronous colorectal liver metastases (CRLM): upfront surgery or neoadjuvant therapy. This study has integrated four available clinical factors - clinicopathological characteristics, gene mutation profiles, imaging findings, and hematological indicators - to create a potentially robust tool aiding clinicians in deciding between upfront surgery and neoadjuvant therapy.
Methods: This retrospective cohort study included individuals diagnosed with resectable synchronous CRLM between 2008 and 2018. The development of prediction nomograms entailed identifying independent prognostic indicators through univariate and multivariate Cox analyses. The accuracy of the predictions was evaluated through calibration curves and the C-index. Furthermore, the clinical effectiveness of the nomograms was assessed using DCA and ROC curves. To enhance accessibility, two web servers were developed to simplify the utilization of the nomograms for an improved user experience.
Results: A total of 386 patients with resectable synchronous CRLM were included. The patients were categorized randomly into a training cohort (n = 270, 70%) and a testing cohort (n = 116, 30%). The nomograms incorporated nine predictors: metastatic tumor count, cN stage, KRAS and BRAF mutation status, age, primary tumor location, neutrophil and platelet counts, and D-Dimer levels. The calibration plots for resectable synchronous CRLM survival predictions showed remarkable consistency. The C-index of OS and DFS prediction models were both above 0.7. And the area under the ROC curve of 1-, 3- and 5-year OS and DFS exceeded 0.7 as well. As demonstrated by the DCA plots, both nomograms exhibit satisfactory clinical effectiveness. A web-based application was developed to demonstrate the practical application of the prediction models.
Conclusion: The personalized web-based predictive models exhibited moderate predictive accuracy in resectable synchronous CRLM. These tools offer valuable assistance to physicians in deciding between upfront surgery and neoadjuvant therapy for resectable synchronous CRLM.
Keywords: CRLM, upfront surgery, neoadjuvant therapy, prognostic models
Introduction
Colorectal cancer (CRC) ranks as the third most common cancer globally, with the second-highest mortality rate[1]. The liver is the most prevalent site for CRC metastases, presenting a challenging prognosis for patients, as evidenced by a 5-year survival rate of less than 10%[1]. Liver resection is the recommended strategy for patients with colorectal liver metastases (CRLM), endorsed clinically and by the National Comprehensive Cancer Network (NCCN) guidelines as the optimal approach for long-term survival and potential cure[2]. Moreover, preoperative therapy offers a spectrum of potential advantages, such as timely intervention for micrometastases, assessment of treatment efficacy, and the avoidance of local therapy for patients exhibiting early disease progression indicators[2-4]. However, the choice between neoadjuvant therapies and upfront surgery remains contentious.
The European Society for Medical Oncology (ESMO)[5] recommends that oncological (prognostic) and technical (surgical) criteria be taken into account when evaluating upfront surgery or neoadjuvant treatment. However, neither ESMO nor NCCN provides definitive screening criteria[2, 5], which results in a heavy reliance on the doctor's expertise. Consequently, there is a growing trend towards risk stratification and the identification of “high-risk” patients[6]. However, persistent issues remain in the field of predictive modeling. Some models only present the variables identified in screening without undergoing validation[7-9] while others focus exclusively on clinicopathological features, overlooking important predictors such as the status of KRAS and BRAF mutations[10].
In addressing the above clinical concerns, this study constructed and validated two evolving preoperative predictive models. It scrutinized nine variables across four domains encompassing clinicopathological features, gene mutation status, imaging evidence, and hematological parameters. By computing patients' survival risk scores, this approach can potentially aid physicians in determining the suitability of upfront surgery for resectable synchronous CRLM.
Materials and Methods
Patient selection
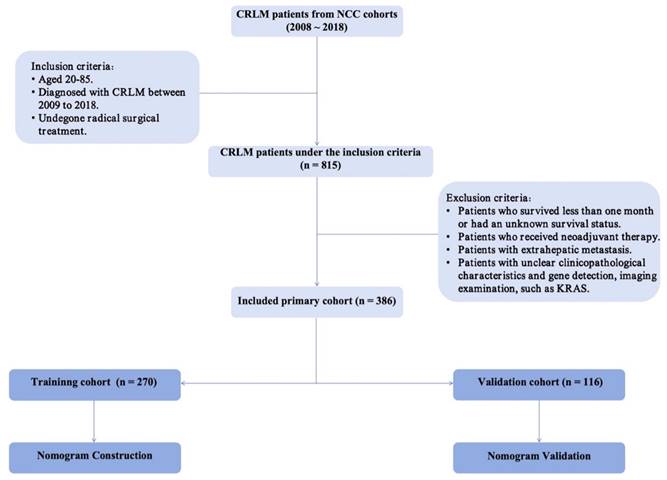
A total of 815 patients diagnosed with CRLM between 2008 and 2018 at the National Cancer Center were included in this retrospective cohort study. Eligibility criteria comprised the following: (1) aged between 20 and 85 years, (2) diagnosis of resectable synchronous CRLM between 2008 and 2018, and (3) undergone radical surgical intervention. Exclusion criteria entailed (1) patients with unknown survival status, (2) individuals who underwent neoadjuvant therapy, (3) patients with extrahepatic metastasis, and (4) patients lacking clear clinicopathological characteristics, imaging examinations, or gene mutation information. A total of 386 patients were recruited for the study. Figure 1 shows the patient screening procedure. The research was retrospectively registered. Patient information was obtained by telephone follow-up (once a year) or review records. The National Cancer Center's Institute Research Medical Ethics Committee approved this study (NCC2021C-125). Following review by the National Cancer Center's Institute Research Medical Ethics Committee, our study was granted an exemption from obtaining signed informed consent.
Prognostic variables
The study gathered data on various parameters including the size of the largest metastatic tumor, number of metastatic tumors, clinical T(cT) and N(cN) stages, KRAS, NRAS, and BRAF statuses, gender, age, body mass index (BMI), American Society of Anesthesiologists (ASA) physical status classification, tumor grade, histological type, primary tumor site, pretreatment carcinoembryonic antigen (CEA) levels, neutrophil, lymphocyte, monocyte, and platelet counts, neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), C-reactive protein (CRP), albumin (Alb), Glasgow Prognostic Score (GPS), red blood cell distribution width (RDW-CV), and D-Dimer levels. The primary outcome of the study was overall survival (OS), defined as the period from the date of diagnosis until death from any cause or the last follow-up. Disease-free survival (DFS) was designated as the secondary endpoint. Patients were divided into two age groups: <70 years old and ≥70 years old. The patients were then categorized based on the following criteria: the largest size of metastatic tumors (<2.25cm and≥2.25cm), the number of metastatic tumors (<3 and≥3), CEA levels (<16.110ng/ml and≥16.110ng/ml), neutrophil count (<3.435*109 and≥3.435*109), lymphocyte count (<2.795*109 and≥2.795*109), monocyte count (<0.545*109 and ≥0.545*109), platelet count (<282.5*109 and≥282.5*109), NLR(<2.677 and≥2.677), PLR(<130.481 and≥130.481), CRP levels (<0.085mg/L and ≥0.085mg/L), albumin levels (<44.95g/dl and ≥44.95g/dl), RDW-CV (<15.35 and ≥15.35), and DD levels (<0.545mg/L and ≥0.545mg/L), based on the optimal cut-off value obtained from the receiver operating characteristic (ROC) curve (refer to Supplementary Figure 1).
Screening procedure for enrolled CRLM patients.
Statistical analysis
Statistical analysis was conducted utilizing the R program, version 4.3.2, available for download at https://www.r-project.org/. The patients were randomly assigned: 70% were designated to the training cohort, and the remaining 30% were allocated to the validation cohort, utilizing the “caret” package in R. Categorical variables are shown as numbers and percentages. Variables with a significance level of P<0.1 were initially selected using univariate Cox regression analysis, followed by multivariate Cox regression analysis to identify independent factors (P<0.05) influencing OS and DFS. Subsequently, two prognostic nomograms were developed, utilizing distinct predictive variables to predict OS and DFS in patients with resectable synchronous CRLM. Dynamic nomogram models were developed using the “rms” and “Dynnom” packages. We employed the Harrell consistency index (C-index), calibration curve, Receiver operating characteristic (ROC) curves and decision curve analysis (DCA) to assess the model's accuracy and appraise its clinical utility. Based on the prediction model, the risk score for each patient was computed, and the optimal cut-off value from the ROC curve was utilized to stratify patients into high-risk and low-risk groups. Subsequently, Kaplan-Meier survival curves were employed to assess the disparities in OS and DFS between these groups. A significance level of p < 0.05 (two-tailed) was applied to all statistical tests.
Results
Patient characteristics
This study included a total of 386 patients with resectable synchronous CRLM, among whom 270 patients (70%) were randomly allocated to the training group, with the remaining 116 patients (30%) assigned to the test group. The median OS and DFS for the entire patient cohort were 40.50 months (IQR, 23.25-61.00) and 25 months (IQR, 9.25-55), respectively. Table 1 displays the detailed data. The majority of patients (57.3%) were under the age of 70. Adenocarcinoma (98.7%) was the predominant pathological type, mostly highly differentiated. Most patients (74.1%) tested negative for the KRAS gene, with similar findings on the NRAS and BRAF genes. Among the sample, 281 individuals (72.8%) had fewer than three metastases. The most common cT stage observed was T3, accounting for 213 cases (55.2%). The left colon was the primary site for 289 patients (74.9%).
Independent influencing factors of OS and DFS were selected
The univariate Cox regression analysis revealed that the number of metastatic tumors, cN stage, KRAS and BRAF gene mutations, patient age, primary tumor site, CEA levels, neutrophil count, platelet count, and D-Dimer levels exhibited a significance level of P <0.1. Hence, these variables demonstrated a significant correlation with patients' OS. The results of the multivariate analysis indicated that the number of metastatic tumors (HR 2.46, P < 0.001), cN stage (P < 0.001), KRAS mutation (HR1.66, P < 0.05), BRAF mutation (HR 4.58, P < 0.001), age (HR 1.62, P < 0.05), primary tumor site (HR 1.61, P < 0.05), neutrophil count (HR 1.80, P < 0.05), platelet count (HR 2.53, P < 0.05), and D-Dimer levels (HR 1.62, P < 0.05) were the factors significantly associated with OS (Table 2). Supplementary Figure 2 presents a forest plot illustrating the HRs and 95% CIs for OS determined through Cox proportional hazards regression analysis.
Characteristics of resectable synchronous CRLM patients
| Characteristics, n (%) | Total cohort (n = 386) | Train cohort (n = 270) | Test cohort (n=116) |
|---|---|---|---|
| The largest metastatic tumor size (cm) | |||
| ≤2.250 | 166 (43.0) | 113 (41.9) | 53 (45.7) |
| >2.250 | 220 (57.0) | 157 (58.1) | 63 (54.3) |
| Number of metastatic tumors | |||
| <3 | 281 (72.8) | 204 (75.6) | 77 (66.4) |
| ≥3 | 105 (27.2) | 66 (24.4) | 39 (33.6) |
| cT stage | |||
| cT1 | 6 (1.6) | 6 (2.2) | 0 (0.0) |
| cT2 | 17 (4.4) | 14 (5.2) | 3 (2.6) |
| cT3 | 213 (55.2) | 147 (54.4) | 66 (56.9) |
| cT4 | 150 (38.9) | 103 (38.1) | 47 (40.5) |
| cN stage | |||
| cN0 | 111 (28.8) | 81 (30.0) | 30 (25.9) |
| cN1 | 146 (37.8) | 95 (35.2) | 51 (44.0) |
| cN2 | 129 (33.4) | 94 (34.8) | 35 (30.2) |
| KRAS gene | |||
| Wild type | 286 (74.1) | 193 (71.5) | 93 (80.2) |
| Mutant | 100 (25.9) | 77 (28.5) | 23 (19.8) |
| NRAS gene | |||
| Wild type | 363 (94.0) | 253 (93.7) | 110 (94.8) |
| Mutant | 23 (6.0) | 17 (6.3) | 6 (5.2) |
| BRAF gene | |||
| Wild type | 369 (95.6) | 259 (95.9) | 110 (94.8) |
| Mutant | 17 (4.4) | 11 (4.1) | 6 (5.2) |
| Sex | |||
| Male | 235 (60.9) | 166 (61.5) | 69 (59.5) |
| Female | 151 (39.1) | 104 (38.5) | 47 (40.5) |
| Age (years) | |||
| ≤70 | 221 (57.3) | 153 (56.7) | 68 (58.6) |
| >70 | 165 (42.7) | 117 (43.3) | 48 (41.4) |
| BMI | |||
| Underweight | 12 (3.1) | 9 (3.3) | 3 (2.6) |
| Normal weight | 199 (51.6) | 139 (51.5) | 60 (51.7) |
| Overweight | 131 (33.9) | 93 (34.4) | 38 (32.8) |
| Obesity | 44 (11.4) | 29 (10.7) | 15 (12.9) |
| ASA | |||
| I-II | 350 (90.7) | 239 (88.5) | 111 (95.7) |
| III-IV | 36 (9.3) | 31 (11.5) | 5 (4.3) |
| Differentiation grade | |||
| Highly | 331 (85.8) | 229 (84.8) | 102 (87.9) |
| Moderately and poorly | 55 (14.2) | 41 (15.2) | 14 (12.1) |
| Pathological type | |||
| Adenocarcinoma | 381 (98.7) | 267 (98.9) | 114 (98.3) |
| Mucinous denocarcinoma | 4 (1.0) | 2 (0.7) | 2 (1.7) |
| High-grade intraepithelial neoplasia | 1 (0.3) | 1 (0.4) | 0 (0.0) |
| Original site | |||
| Left semicolon | 289 (74.9) | 202 (74.8) | 87 (75.0) |
| Right semicolon | 97 (25.1) | 68 (25.2) | 29 (25.0) |
| CEA (ng/ml) | |||
| ≤ 16.110 | 245 (63.5) | 172 (63.7) | 73 (62.9) |
| > 16.110 | 141 (36.5) | 98 (36.3) | 43 (37.1) |
| Neutrophil count (*109/L) | |||
| ≤ 3.435 | 154 (39.9) | 110 (40.7) | 44 (37.9) |
| > 3.435 | 232 (60.1) | 160 (59.3) | 72 (62.1) |
| Lymphocyte count (*109/L) | |||
| ≤ 2.795 | 351 (90.9) | 246 (91.1) | 105 (90.5) |
| > 2.795 | 35 (9.1) | 24 (8.9) | 11 (9.5) |
| Monocyte count (*109/L) | |||
| ≤ 0.545 | 290 (75.1) | 204 (75.6) | 86 (74.1) |
| > 0.545 | 96 (24.9) | 66 (24.4) | 30 (25.9) |
| Platelet count (*109/L) | |||
| ≤ 282.510 | 302 (78.2) | 213 (78.9) | 89 (76.7) |
| > 282.510 | 84 (21.8) | 57 (21.1) | 27 (23.3) |
| NLR | |||
| ≤2.677 | 267 (69.2) | 188 (69.6) | 79 (68.1) |
| >2.677 | 119 (30.8) | 82 (30.4) | 37 (31.9) |
| PLR | |||
| ≤130.481 | 204 (52.8) | 150 (55.6) | 54 (46.6) |
| >130.481 | 182 (47.2) | 120 (44.4) | 62 (53.4) |
| CRP (mg/L) | |||
| ≤ 0.085 | 159 (41.2) | 100 (37.0) | 59 (50.9) |
| > 0.085 | 227 (58.8) | 170 (63.0) | 57 (49.1) |
| Albumin (g/L) | |||
| ≤ 44.950 | 269 (69.7) | 183 (67.8) | 86 (74.1) |
| > 44.950 | 117 (30.3) | 87 (32.2) | 30 (25.9) |
| RDW-CV | |||
| ≤ 15.350 % | 291 (75.4) | 205 (75.9) | 86 (74.1) |
| > 15.350 % | 95 (24.6) | 65 (24.1) | 30 (25.9) |
| D-Dimer (mg/L) | |||
| ≤ 0.545 | 257 (66.6) | 180 (66.7) | 77 (66.4) |
| > 0.545 | 129 (33.4) | 90 (33.3) | 39 (33.6) |
| GPS | |||
| CRP≤10mg/L & Alb≥35g/L | 374 (96.9) | 262 (97.0) | 112 (96.6) |
| CRP≤10mg/L & Alb<35g/L CRP>10mg/L & Alb≥35g/L | 12 (3.1) | 8 (3.0) | 4 (3.4) |
| CRP>10mg/L & Alb<35g/L | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Cox regression analysis of OS in the training cohort
| Factor | Univariate analyses | Multivariate analyses | ||
|---|---|---|---|---|
| P value | HR (95%CI) | P value | HR (95%CI) | |
| The largest metastatic tumor size (cm) | 0.457 | |||
| ≤2.250 | Reference | |||
| >2.250 | 0.86 (0.58-1.28) | |||
| Number of metastatic tumors | <0.001 | <0.001 | ||
| <3 | Reference | Reference | ||
| ≥3 | 2.6 (1.69-4.01) | 2.46 (1.54-3.92) | ||
| cT stage | ||||
| cT1 | Reference | |||
| cT2 | 0.520 | 0.45 (0.04-5.02) | ||
| cT3 | 0.955 | 0.95 (0.13-6.86) | ||
| cT4 | 0.790 | 0.79 (0.18-9.51) | ||
| cN stage | ||||
| cN0 | Reference | |||
| cN1 | 0.057 | 1.72 (0.98-3.01) | 0.590 | 1.18 (0.65-2.14) |
| cN2 | 0.001 | 3.05 (1.77-5.26) | <0.001 | 3.18 (1.82-5.57) |
| KRAS gene | 0.005 | 0.029 | ||
| Wild type | Reference | Reference | ||
| Mutant | 1.85 (1.21-2.83) | 1.66 (1.05-2.71) | ||
| NRAS gene | 0.391 | |||
| Wild type | Reference | |||
| Mutant | 1.48 (0.6-3.66) | |||
| BRAF gene | <0.001 | <0.001 | ||
| Wild type | Reference | Reference | ||
| Mutant | 5.46 (2.36-12.66) | 4.58 (1.88-11.17) | ||
| Sex | 0.538 | |||
| Male | Reference | |||
| Female | 1.14 (0.76-1.7) | |||
| Age (years) | 0.002 | 0.024 | ||
| ≤70 | Reference | Reference | ||
| >70 | 1.89 (1.27-2.83) | 1.62 (1.07-2.46) | ||
| BMI | ||||
| Underweight | Reference | |||
| Normal weight | 0.285 | 2.16 (0.53-8.85) | ||
| Overweight | 0.556 | 1.54 (0.37-6.48) | ||
| Obesity | 0.359 | 2.04 (0.45-9.32) | ||
| ASA | 0.143 | |||
| I-II | Reference | |||
| III-IV | 0.54 (0.24-1.23) | |||
| Differentiation grade | 0.648 | |||
| Highly | Reference | |||
| Moderately and poorly | 1.15 (0.63-2.12) | |||
| Pathological type | ||||
| Adenocarcinoma | Reference | |||
| Mucinous denocarcinoma | 0.996 | 0 (0-Inf) | ||
| High-grade intraepithelial neoplasia | 0.998 | 0 (0-Inf) | ||
| Original site | 0.039 | 0.046 | ||
| Left semicolon | Reference | Reference | ||
| Right semicolon | 1.59 (1.02-2.47) | 1.61 (1.01-2.57) | ||
| CEA (ng/ml) | 0.016 | 0.068 | ||
| ≤ 16.110 | Reference | Refernece | ||
| > 16.110 | 1.65 (1.1-2.49) | 1.49 (0.97-2.28) | ||
| Neutrophil count (*109 /L) | 0.089 | 0.010 | ||
| ≤ 3.435 | Reference | Reference | ||
| > 3.435 | 1.46 (0.94-2.24) | 1.80 (1.15-2.82) | ||
| Lymphocyte count (*109 /L) | 0.184 | |||
| ≤ 2.795 | Reference | |||
| > 2.795 | 0.57 (0.25-1.31) | |||
| Monocyte count (*109 /L) | 0.215 | |||
| ≤ 0.545 | Reference | |||
| > 0.545 | 1.31 (0.85-2.01) | |||
| Platelet count (*109 /L) | 0.005 | 0.003 | ||
| ≤ 282.510 | Reference | Reference | ||
| > 282.510 | 2.32 (1.29-4.16) | 2.53 (1.37-4.68) | ||
| NLR | 0.848 | |||
| ≤2.677 | Reference | |||
| >2.677 | 1.04 (0.67-1.61) | |||
| PLR | 0.947 | |||
| ≤130.481 | Reference | |||
| >130.481 | 0.99 (0.66-1.48) | |||
| CRP (mg/L) | 0.176 | |||
| ≤ 0.085 | Reference | |||
| > 0.085 | 1.33 (0.88-2.01) | |||
| Albumin (g/L) | 0.339 | |||
| ≤ 44.950 | Reference | |||
| > 44.950 | 0.61 (0.22-1.67) | |||
| RDW-CV | 0.316 | |||
| ≤ 15.35 % | Reference | |||
| > 15.35 % | 1.26 (0.8-2) | |||
| D-Dimer (mg/L) | 0.059 | 0.034 | ||
| ≤ 0.545 | Reference | Reference | ||
| > 0.545 | 1.49 (0.98-2.25) | 1.62 (1.04-2.51) | ||
| GPS | ||||
| CRP≤10mg/L & Alb≥35g/L | Reference | |||
| CRP≤10mg/L & Alb<35g/L CRP>10mg/L & Alb≥35g/L | 0.533 | 1.38 (0.5-3.75) | ||
| CRP>10mg/L & Alb<35g/L | NULL | NULL | ||
In predicting DFS, factors such as the number of metastatic tumors, cN stage, KRAS and BRAF mutations, patient age, primary tumor site, CEA levels, neutrophil count, monocyte count, platelet count, and D-Dimer levels were identified as significant predictors for DFS using univariate analysis. The subsequent multivariate analysis revealed that the number of metastatic tumors (HR 2.48, P < 0.001), cN stage (HR 3.32, P < 0.001), KRAS (HR 1.74, P < 0.05), BRAF (HR 2.81, P < 0.001), age (HR, P < 0.05), original site (HR 1.83, P < 0.05), neutrophil count (HR 1.61, P < 0.05), platelet count (HR 2.58, P < 0.05), D-Dimer (HR 1.62, P < 0.05) were identified as independent predictors. These findings are presented in Table 3. Moreover, a forest plot from the multivariate regression analysis for DFS was generated in Supplementary Figure 3.
Cox regression analysis of DFS in the training cohort
| Factor | Univariate analyses | Multivariate analyses | ||
|---|---|---|---|---|
| P value | HR (95%CI) | P value | HR (95%CI) | |
| The largest metastatic tumor size (cm) | 0.744 | |||
| ≤2.250 | Reference | |||
| >2.250 | 0.94 (0.63-1.4) | |||
| Number of metastatic tumors | <0.001 | <0.001 | ||
| <3 | Reference | Reference | ||
| ≥3 | 2.47 (1.6-3.8) | 2.48 (1.54-3.92) | ||
| cT stage | ||||
| cT1 | Reference | |||
| cT2 | 0.661 | 0.58 (0.05-6.45) | ||
| cT3 | 0.838 | 1.23 (0.17-8.92) | ||
| cT4 | 0.583 | 1.74 (0.24-12.65) | ||
| cN stage | ||||
| cN0 | Reference | |||
| cN1 | 0.020 | 1.94 (1.11-3.4) | 0.193 | 1.53 (0.82-2.63) |
| cN2 | <0.001 | 3.14 (1.82-5.41) | <0.001 | 3.32 (1.89-5.92) |
| KRAS gene | 0.002 | 0.028 | ||
| Wild type | Reference | Reference | ||
| Mutant | 1.95 (1.27-2.98) | 1.74 (1.06-2.67) | ||
| NRAS gene | 0.643 | |||
| Wild type | Reference | |||
| Mutant | 1.24 (0.5-3.05) | |||
| BRAF gene | <0.001 | 0.032 | ||
| Wild type | Reference | Reference | ||
| Mutant | 5.85 (2.52-13.57) | 2.81 (1.09-7.14) | ||
| Sex | 0.752 | |||
| Male | Reference | |||
| Female | 1.07 (0.71-1.6) | |||
| Age (years) | 0.004 | 0.022 | ||
| ≤70 | Reference | Reference | ||
| >70 | 1.8 (1.2-2.69) | 1.62 (1.07-2.51) | ||
| BMI | ||||
| Underweight | Reference | |||
| Normal weight | 0.348 | 1.96 (0.48-8.05) | ||
| Overweight | 0.725 | 1.29 (0.31-5.45) | ||
| Obesity | 0.570 | 1.55 (0.34-7.09) | ||
| ASA | 0.154 | |||
| I-II | Reference | |||
| III-IV | 0.55 (0.24-1.25) | |||
| Differentiation grade | 0.639 | |||
| Highly | Reference | |||
| Moderately and poorly | 1.16 (0.63-2.12) | |||
| Original site | 0.037 | 0.021 | ||
| Left semicolon | Reference | Reference | ||
| Right semicolon | 1.6 (1.03-2.48) | 1.83 (1.09-2.79) | ||
| CEA (ng/ml) | 0.006 | 0.069 | ||
| ≤ 16.110 | Reference | Refernece | ||
| > 16.110 | 1.78 (1.18-2.69) | 1.51 (0.97-2.27) | ||
| Neutrophil count (*109 /L) | 0.041 | 0.044 | ||
| ≤ 3.435 | Reference | Reference | ||
| > 3.435 | 1.43 (1.03-2.42) | 1.61 (1.01-2.52) | ||
| Lymphocyte count (*109 /L) | 0.165 | |||
| ≤ 2.795 | Reference | |||
| > 2.795 | 0.56 (0.24-1.27) | |||
| Monocyte count | 0.088 | 0.197 | ||
| ≤ 0.545 | Reference | Reference | ||
| > 0.545 | 1.45 (0.95-2.23) | 1.41 (0.85-2.14) | ||
| Platelet count | 0.005 | 0.003 | ||
| ≤ 282.5 | Reference | Reference | ||
| > 282.5 | 2.33 (1.29-4.18) | 2.58 (1.38-4.70) | ||
| NLR | 0.909 | |||
| ≤2.677 | Reference | |||
| >2.677 | 0.97 (0.63-1.51) | |||
| PLR | 0.841 | |||
| ≤130.481 | Reference | |||
| >130.481 | 0.96 (0.64-1.44) | |||
| CRP (mg/L) | 0.190 | |||
| ≤ 0.085 | Reference | |||
| > 0.085 | 1.32 (0.87-1.99) | |||
| Albumin (g/L) | 0.218 | |||
| ≤ 44.950 | Reference | |||
| > 44.95 0 | 0.53 (0.2-1.45) | |||
| RDW-CV | 0.425 | |||
| ≤ 15.350 % | Reference | |||
| > 15.350 % | 1.2 (0.76-1.9) | |||
| D-Dimer (mg/L) | 0.055 | 0.041 | ||
| ≤ 0.545 | Reference | Reference | ||
| > 0.545 | 1.5 (0.99-2.27) | 1.62 (1.02-2.48) | ||
| GPS | ||||
| CRP≤10mg/L & Alb≥35g/L | Reference | |||
| CRP≤10mg/L & Alb<35g/L CRP>10mg/L & Alb≥35g/L | 0.358 | 1.6 (0.59-4.37) | ||
| CRP>10mg/L & Alb<35g/L | NULL | NULL | ||
Development of nomograms for OS and DFS
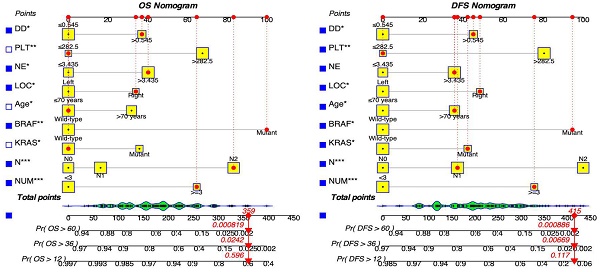
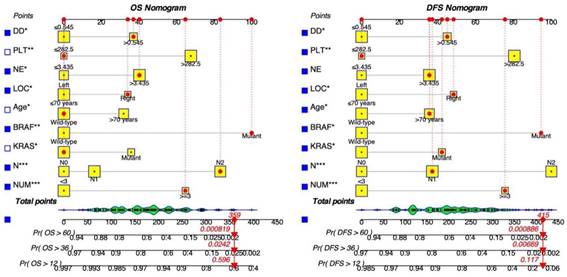
Key screening indicators, including the number of metastatic tumors, cN stage, mutations in KRAS and BRAF genes, patient age, primary tumor site, neutrophil count, platelet count, and D-Dimer levels, were integrated into the dynamic prediction model to forecast OS and DFS in patients with resectable synchronous CRLM. Figure 2 illustrates the process of determining a patient's score for each variable by intersecting a vertical line with the factor value score line. The cumulative total score corresponds to various survival rates. This visual model enables an intuitive assessment of the likelihood of 1-year, 3-year, and 5-year survival as well as disease-free survival.
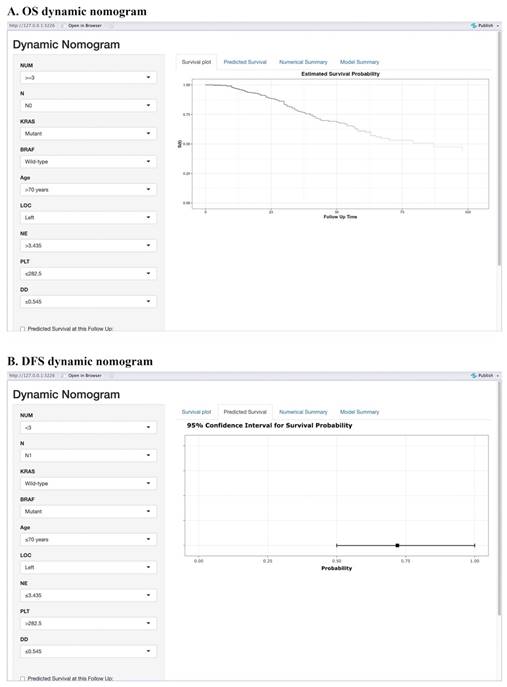
Moreover, we have created an interactive online application featuring the generated nomograms (Refer to Figure 3). The hyperlinks (https://alxpcun.shinyapps.io/OS-nomogram/and https://alxpcun.shinyapps.io/DFS-nomogram/)are accessible for navigation.
Validation of the nomograms
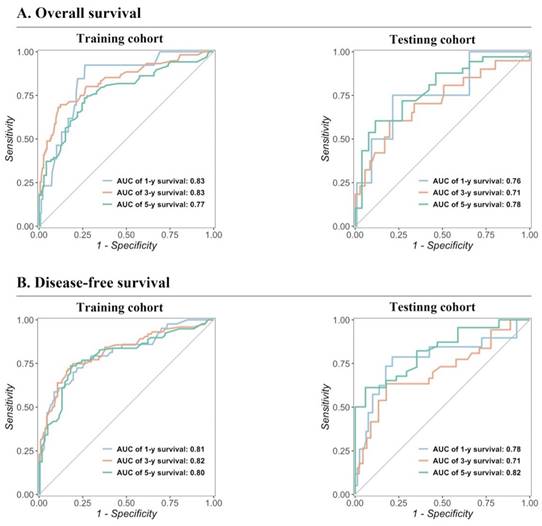
The predictive performance of the models in both the training and testing cohorts was evaluated through calibration plots, C-index, AUC, and DCA. Initially, calibration curves were generated to compare the projected 1-, 3-, and 5-year OS and DFS probabilities from the nomogram models with the actual outcomes of resectable synchronous CRLM patients in the training and testing sets. The results demonstrated strong concordance between the predicted probabilities and the observed outcomes, validating a high level of accuracy in the predictions, as depicted in Figure 4. The nomogram exhibited substantial precision in survival prediction, with C-index values of 0.773 ± 0.05 and 0.764 ± 0.053 for OS and DFS, respectively, in the training cohort. In the testing group, the C-index values were 0.747 ± 0.085 and 0.741 ± 0.082 for OS and DFS, respectively. ROC curves were constructed to evaluate the sensitivity and specificity of the nomogram prediction models. The training cohort displayed AUC values of 0.83, 0.83, and 0.77 for OS at 1, 3, and 5 years, respectively, as well as AUC values of 0.81, 0.82, and 0.80 for DFS at the corresponding time points. Remarkably, the testing cohort displayed AUC values of 0.76, 0.71, and 0.78 for OS and 0.78, 0.71, and 0.82 for DFS at 1, 3, and 5 years respectively, as illustrated in Figure 5. In summary, our data validated the high sensitivity and specificity of our nomogram models.
Dynamic nomograms for predicting the prognosis of patients with resectable synchronous CRLM. Distinct variables are associated with specific scores, and the cumulative scores correspond to the 1-year, 3-year, and 5-year survival rates.
Web-based prognostic nomogram for patients with resectable synchronous CRLM. Available at: https://alxpcun.shinyapps.io/OS-nomogram/ and https://alxpcun.shinyapps.io/DFS-nomogram/.
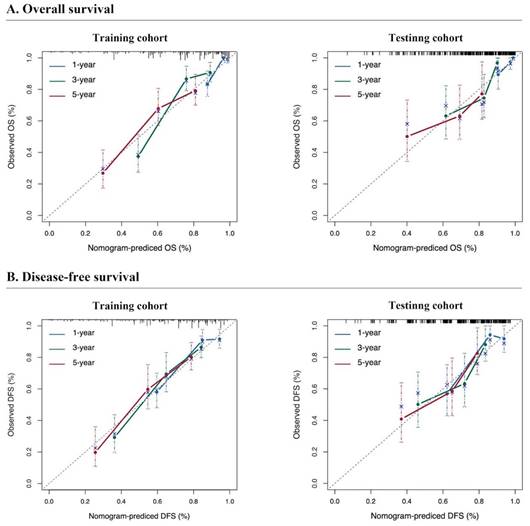
Calibration curves for predicting the survival of resectable synchronous CRLM patients. The calibration curve of the prediction model closely approximates the reference line, signifying a strong alignment between the predicted and actual probabilities.
Validation of the prognostic nomograms using ROC curves.
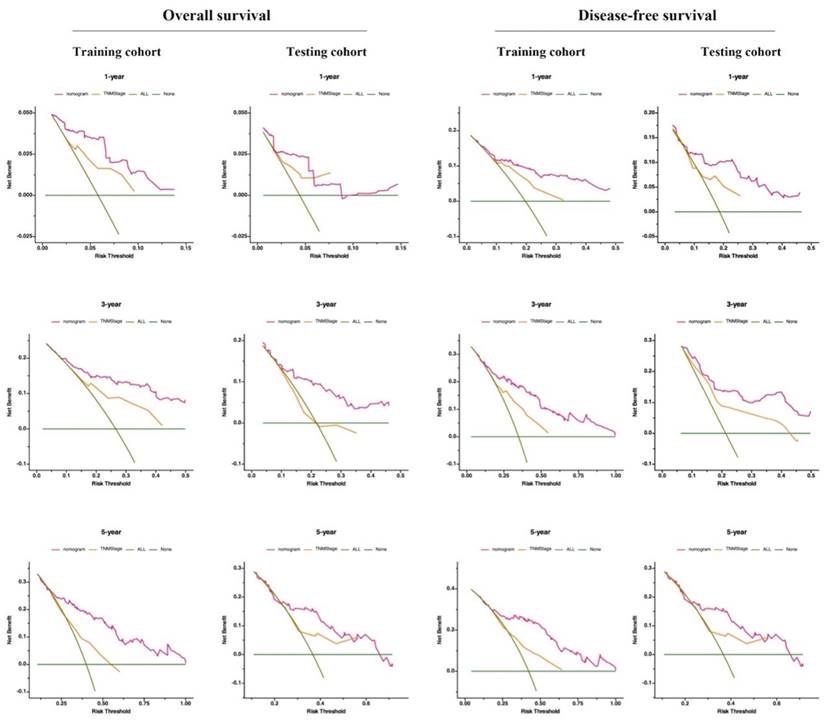
Validation of the prognostic nomograms using DCA curves. ALL, all patients died or relapsed; none, no patients died or relapsed. The net benefit rate of the nomogram curve surpasses those of the TNM stage and extreme curve, highlighting the substantial practical utility of prediction models.
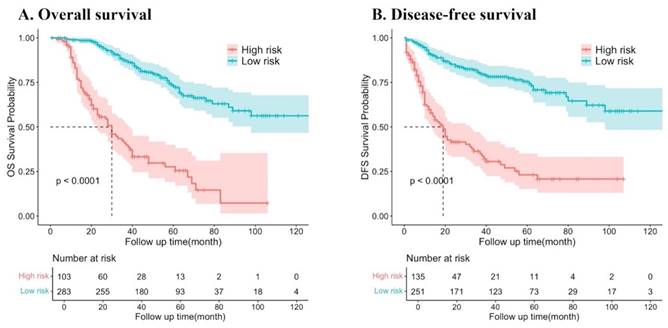
Kaplan‒Meier survival curves of resectable synchronous CRLM patients at different risk factors for death.
Additionally, DCA serves as a widely adopted method for evaluating the clinical usefulness of nomograms. The nomograms surpass the conventional TNM staging system, demonstrating superior predictive capabilities for mortality risk, as depicted in Figure 6. These results emphasize the considerable practical importance of nomograms in predicting OS and DFS for patients diagnosed with resectable synchronous CRLM.
Risk status of patients stratified by the prediction models
The predictor variable scores were calculated using the nomogram and then totaled to determine the cumulative scores for each patient. Patients with resectable synchronous CRLM were stratified into low- and high-risk categories based on their nomogram scores. The threshold score was set at 295.4 points for DFS and 283.5 points for OS on the nomogram. Patients exceeding the threshold were classified as high risk. Survival analysis revealed a significantly lower probability of DFS and OS in the high-risk group compared to the low-risk group (both P < 0.001). These results indicate that the nomograms utilized in this study can effectively stratify the risk for resectable synchronous CRLM patients (Figure 7).
Discussion
NCCN and ESMO guidelines endorse surgical intervention or perioperative (neoadjuvant plus postoperative) systemic therapy for patients with resectable synchronous colorectal liver metastases (CRLM)[2, 5]. However, the optimal sequence for administering systemic therapy and resection remains uncertain. The decision between neoadjuvant therapy and upfront surgery for resectable synchronous CRLM varies greatly among medical centers and is largely subjective. Present predictive models for resectable synchronous CRLM predominantly focus on postoperative survival prognostication rather than assisting in the choice between upfront surgery or neoadjuvant therapy. They also disregard vital hematological and genetic factors, such as neutrophil count, platelet count, and KRAS status, which are known to significantly influence patient survival[11, 12]. Thus, further research is imperative to investigate the factors influencing the prolonged survival of resectable synchronous CRLM patients and to create predictive models for optimizing treatment selection.
In order to enhance the comprehensiveness and accuracy of the model, this study introduced four indices - clinicopathological features, genetic status, imaging, and hematological factors - for the first time. Subsequently, univariate and multivariate Cox regression analyses were conducted using comprehensive clinical data to identify independent risk variables for the prognosis of OS and DFS in patients with resectable synchronous CRLM. Nine prognostic factors were then identified: metastatic tumor count, cN stage, KRAS and BRAF status, patient age, primary tumor location, neutrophil and platelet counts, and D-Dimer level. Furthermore, an interactive online tool was developed to expedite clinical decision-making based on the provided nomograms. The calibration of the nomograms demonstrated strong performance, with the OS and DFS models exhibiting a C-index and AUC greater than 0.7, indicating their robust discriminative ability. Additionally, DCA illustrated that our novel nomogram models offered superior clinical utility compared to the traditional TNM staging system across various threshold probabilities. These results suggest that our nomograms could aid clinicians in deciding between upfront surgery and neoadjuvant therapy.
In recent years, various studies have highlighted the significance of the primary tumor site, lymph node metastasis status, age, and the number of metastatic tumors as key prognostic factors for patients with resectable synchronous CRLM, leading to their incorporation into clinical prediction models[13, 14]. Our research aligns with these findings. Although there are differences in how these variables are grouped, they consistently emerge as independent risk factors affecting the outcomes of resectable synchronous CRLM patients. Busiman et al.[15] integrated several factors including age, gender, location and nodal status of CRC, disease-free interval, number and size of CRLM, preoperative CEA levels, resection margin, presence of extrahepatic disease, KRAS and BRAF mutation status, histopathological growth pattern, perioperative systemic chemotherapy, and perioperative hepatic arterial infusion pump (HAIP) chemotherapy into their clinical prediction model using Cox regression analysis, yielding satisfactory predictive performance. It is noteworthy that their model focused on predicting the 10-year survival post-surgery for patients with CRLM, rather than aiding in preoperative surgical decision-making, and did not consider the predictive value of hematological factors. These limitations are also prevalent in other related studies[16-18].
Genetic factors, such as the status of BRAF and KRAS, significantly impact the treatment recommendations for patients with resectable synchronous CRLM[11, 19]. Previous models were limited by data availability, hindering the incorporation of all relevant variables. Our models emphasize the crucial roles of KRAS and BRAF in predictive modeling. Mutations in KRAS and BRAF promote cancer progression by enhancing angiogenesis, influencing cell motility and adhesion, and triggering aggressive biological traits in cancer cells[20, 21]. As a result, patients demonstrate significantly decreased OS and early tumor recurrence. Our findings are consistent with the study by Huang et al.[22], confirming that KRAS and BRAF mutations act independently as prognostic risk factors in resectable synchronous CRLM patients. While the mutation status significantly influenced the outcome in the prediction model, the mutation rates of KRAS and BRAF were notably low, diminishing their clinical predictive value[23]. Additionally, increasing research supports the use of hematological markers such as neutrophil count, platelet count, and NLR in predicting the presence of resectable synchronous CRLM[24-26], thereby strengthening prediction models. Neutrophils play a significant role in the progression and spread of CRLM, underscoring their clinical relevance. Neutrophils secrete interleukin-1 (IL-1) and tumor necrosis factor, stimulating the production of granulocyte-colony stimulating factors, resulting in elevated levels of vascular endothelial growth factor, antiapoptotic markers, and transcription factors[12]. Nevertheless, further research is necessary to fully elucidate the underlying mechanism. In a study by Pedrazzan[22] et al., it was revealed that patients with a high platelet count experience reduced long-term survival rates. The study further emphasized that a high platelet count independently acts as a prognostic factor for patients who underwent potentially curative resections, supporting our own findings. Despite these similarities, the exact mechanism remains uncertain, with several potential explanations requiring empirical validation[27-30]. Multiple studies have demonstrated that D-Dimer affects cellular communication systems, promoting increased cell proliferation and initiating angiogenesis[31]. Additionally, it has been observed to facilitate the progression and spread of malignancies by enhancing tumor cell attachment to endothelial cells[32]. Chen et al.[32] have shown a correlation between elevated D-Dimer levels and decreased OS and DFS, consistent with our own research results. Furthermore, we incorporated D-Dimer levels into the clinical prediction model.
Our clinical predictive models offer several advantages. Firstly, patients with resectable synchronous CRLM were classified into high-risk and low-risk groups based on optimal cutoff values. Substantial differences in OS and DFS were noted across the risk categories. By using a risk score, physicians can tailor treatment strategies for resectable synchronous CRLM patients, aiding in the decision-making process between upfront surgical intervention and neoadjuvant therapy. Secondly, our nomograms incorporate a range of factors, including clinicopathological characteristics, genetic status, radiological findings, and preoperative blood parameters, providing a comprehensive evaluation. Moreover, the prognostic factors considered are readily available through routine preoperative examinations and blood tests, enabling healthcare professionals to personalize treatment plans for each patient.
Nevertheless, our study does have limitations. The retrospective design of the research poses a constraint that could lead to recall bias. Implementing a multicenter prospective cohort study would offer further evidence to substantiate our model. Moreover, to mitigate selection bias, we excluded patients with ambiguous data in their variables. For additional assurance, it may be necessary to validate the nomograms employed in this study through confirmation from cohorts at diverse medical centers.
Conclusion
Our study was groundbreaking as it identified nine preoperatively accessible indicators from four critical domains: clinicopathological features, gene status, imaging, and hematology factors. These indicators were instrumental in developing a predictive model specifically tailored for direct surgery in resectable synchronous CRLM patients. The model has demonstrated exceptional accuracy in guiding treatment selection. In summary, we present a novel predictive model that estimates the survival rates of resectable synchronous CRLM patients who have undergone direct surgery using easily obtainable clinical indicators. This tool is anticipated to significantly aid clinicians in treatment decision-making processes between upfront surgery and neoadjuvant therapy.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
We appreciate the trust and cooperation of the patients and their families.
Funding
This study was supported by Beijing Hope Run Special Fund of the Cancer Foundation of China (LC2022L01).
Author contributions
Dedi Jiang: design of the work, the acquisition, analysis, interpretation of data; Yujuan Jiang: conception, the acquisition, analysis; Jinghua Chen: data curation, conception, interpretation of data; Heting Feng: data curation, the acquisition, analysis; Zixing Zhu: data curation; Jun Jiang: have drafted the work or substantively revised it; Fan Wu: have drafted the work or substantively revised it; Jianwei Liang: conception, have drafted the work or substantively revised it. All the authors have read and agreed to the published version of the manuscript.
Ethics approval and consent to participate
This retrospective study meeting the ethical standards of the Helsinki Declarations was approved by the National Cancer Center's Institute Research Medical Ethics Committee (Reference number: NCC2021C-125). This study did not breach personal privacy or commercial interests and retrospectively obtained patient diagnosis and treatment data from our hospital's medical system, posing minimal risk to patients. Following review by the National Cancer Center's Institute Research Medical Ethics Committee, our study was granted an exemption from obtaining signed informed consent.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-63
2. Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK. et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:329-59
3. Araujo RLC, Fonseca LG, Silva RO, Linhares MM, Uson Junior PLS. Molecular profiling and patient selection for the multimodal approaches for patients with resectable colorectal liver metastases. Hepatobiliary Surg Nutr. 2024;13:273-92
4. Sebagh M, Allard MA, Cunha AS, Ruiz A, Araujo R, Lemoine A. et al. A proposed new method for assessing the pathological response to chemotherapy in resected colorectal liver metastases. Br J Cancer. 2014;111:470-6
5. Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D. et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386-422
6. Kokkinakis S, Ziogas IA, Llaque Salazar JD, Moris DP, Tsoulfas G. Clinical Prediction Models for Prognosis of Colorectal Liver Metastases: A Comprehensive Review of Regression-Based and Machine Learning Models. Cancers (Basel). 2024;16:1645
7. Chen F-L, Wang Y-Y, Liu W, Xing B-C. Prognostic factors in colorectal liver metastases patients with various tumor numbers treated by liver resection: a single-center, retrospective study. World J Surg Oncol. 2022;20:237
8. Fang C, Huang Y, Chen C, Nie D, Lin J, Xiao Z. et al. The Prognostic Value of Serum Apolipoprotein A-I Level and Neutrophil-to-Lymphocyte Ratio in Colorectal Cancer Liver Metastasis. J Oncol. 2022;2022:9149788
9. Qin S, Hu H, Cui R, Lin J, Liu Y, Wang Y. et al. A prognostic nomogram for intrahepatic progression-free survival in patients with colorectal liver metastases after ultrasound-guided percutaneous microwave ablation. Int J Hyperthermia. 2022;39:144-54
10. He M, Jia Z, Hu L, Wu H. Development and validation of a nomogram to predict which patients with colorectal cancer liver metastases would benefit from primary tumor resection. Int J Colorectal Dis. 2023;38:144
11. Passiglia F, Bronte G, Bazan V, Galvano A, Vincenzi B, Russo A. Can KRAS and BRAF mutations limit the benefit of liver resection in metastatic colorectal cancer patients? A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2016;99:150-7
12. Lin N, Li J, Yao X, Zhang X, Liu G, Zhang Z. et al. Prognostic value of neutrophil-to-lymphocyte ratio in colorectal cancer liver metastasis: A meta-analysis of results from multivariate analysis. Int J Surg. 2022;107:106959
13. Chen D, Li Q, Yu H. Prognosis of resectable colorectal liver metastases after surgery associated with pathological features of primary tumor. Front Oncol. 2023;13:1181522
14. Amygdalos I, Müller-Franzes G, Bednarsch J, Czigany Z, Ulmer TF, Bruners P. et al. Novel machine learning algorithm can identify patients at risk of poor overall survival following curative resection for colorectal liver metastases. J Hepatobiliary Pancreat Sci. 2023;30:602-14
15. Buisman FE, Giardiello D, Kemeny NE, Steyerberg EW, Hoppener DJ, Galjart B. et al. Predicting 10-year survival after resection of colorectal liver metastases; an international study including biomarkers and perioperative treatment. Eur J Cancer. 2022;168:25-33
16. Bai L, Yan XL, Lu YX, Meng Q, Rong YM, Ye LF. et al. Circulating Lipid- and Inflammation-Based Risk (CLIR) Score: A Promising New Model for Predicting Outcomes in Complete Colorectal Liver Metastases Resection. Ann Surg Oncol. 2022;29:4308-23
17. Reijonen P, Nordin A, Savikko J, Poussa T, Arola J, Isoniemi H. Histopathological Helsinki score of colorectal liver metastases predicts survival after liver resection. APMIS. 2023;131:249-61
18. Sasaki K, Margonis GA, Moro A, Wang J, Wagner D, Gagniere J. et al. Nontumor related risk score: A new tool to improve prediction of prognosis after hepatectomy for colorectal liver metastases. Surgery. 2022;171:1580-7
19. Brudvik KW, Kopetz SE, Li L, Conrad C, Aloia TA, Vauthey JN. Meta-analysis of KRAS mutations and survival after resection of colorectal liver metastases. Br J Surg. 2015;102:1175-83
20. Hewitt DB, Brown ZJ, Pawlik TM. The Role of Biomarkers in the Management of Colorectal Liver Metastases. Cancers (Basel). 2022;14:4602
21. Hondo N, Kitazawa M, Koyama M, Nakamura S, Tokumaru S, Miyazaki S. et al. MEK inhibitor and anti-EGFR antibody overcome sotorasib resistance signals and enhance its antitumor effect in colorectal cancer cells. Cancer Lett. 2023;567:216264
22. Huang CJ, Teng HW, Chien CC, Lin JK, Yang SH. Prognostic significance of C-reactive protein polymorphism and KRAS/BRAF in synchronous liver metastasis from colorectal cancer. PLoS One. 2014;8:e65117
23. Chen Q, Chen J, Deng Y, Bi X, Zhao J, Zhou J. et al. Personalized prediction of postoperative complication and survival among Colorectal Liver Metastases Patients Receiving Simultaneous Resection using machine learning approaches: A multi-center study. Cancer Letters. 2024;593:216967
24. Chen Q, Mao R, Zhao J, Bi X, Li Z, Huang Z. et al. Nomograms incorporating preoperative RDW level for the prediction of postoperative complications and survival in colorectal liver metastases after resection. Ann Palliat Med. 2021;10:4143-58
25. Erstad DJ, Taylor MS, Qadan M, Axtell AL, Fuchs BC, Berger DL. et al. Platelet and neutrophil to lymphocyte ratios predict survival in patients with resectable colorectal liver metastases. Am J Surg. 2020;220:1579-85
26. Kanikarla Marie P, Fowlkes NW, Afshar-Kharghan V, Martch SL, Sorokin A, Shen JP. et al. The Provocative Roles of Platelets in Liver Disease and Cancer. Front Oncol. 2021;11:643815
27. Kawai K, Watanabe T. Colorectal cancer and hypercoagulability. Surg Today. 2014;44:797-803
28. Poggi A, Stella M, Donati MB. The importance of blood cell-vessel wall interactions in tumour metastasis. Baillieres Clin Haematol. 1993;6:731-52
29. Placke T, Kopp HG, Salih HR. The wolf in sheep's clothing: Platelet-derived "pseudo self" impairs cancer cell "missing self" recognition by NK cells. Oncoimmunology. 2012;1:557-9
30. Seretis C, Youssef H, Chapman M. Hypercoagulation in colorectal cancer: what can platelet indices tell us? Platelets. 2015;26:114-8
31. Dupuy E, Hainaud P, Villemain A, Bodevin-Phedre E, Brouland JP, Briand P. et al. Tumoral angiogenesis and tissue factor expression during hepatocellular carcinoma progression in a transgenic mouse model. J Hepatol. 2003;38:793-802
32. Buller HR, van Doormaal FF, van Sluis GL, Kamphuisen PW. Cancer and thrombosis: from molecular mechanisms to clinical presentations. J Thromb Haemost. 2007;5(Suppl 1):246-54
Author contact
![]() Corresponding authors: Liang Jianwei, No. 17 Panjiayuan Nanli, Chaoyang District, Beijing 100021, China; Email: liangjw1976com; ORCID: 0000-0003-2567-5429. Wu Fan, No. 17 Panjiayuan Nanli, Chaoyang District, Beijing 100021, China; Email: 13439005727com. Jiang Jun, No. 17 Panjiayuan Nanli, Chaoyang District, Beijing 100021, China; Email: 519111066com.
Corresponding authors: Liang Jianwei, No. 17 Panjiayuan Nanli, Chaoyang District, Beijing 100021, China; Email: liangjw1976com; ORCID: 0000-0003-2567-5429. Wu Fan, No. 17 Panjiayuan Nanli, Chaoyang District, Beijing 100021, China; Email: 13439005727com. Jiang Jun, No. 17 Panjiayuan Nanli, Chaoyang District, Beijing 100021, China; Email: 519111066com.

 Global reach, higher impact
Global reach, higher impact