Impact Factor
ISSN: 1837-9664
J Cancer 2025; 16(5):1684-1693. doi:10.7150/jca.105024 This issue Cite
Review
Malignant Transformation of Meningiomas
1. Department of Neurosurgery, Huashan Hospital, Shanghai Medical College, Fudan University, Shanghai, China.
2. Institute of Neurosurgery, Fudan University, Shanghai, China.
3. Shanghai Key Laboratory of Brain Function Restoration and Neural Regeneration, Fudan University, Shanghai, China.
4. Department of Critical Care Medicine, Huashan Hospital, Shanghai Medical College, Fudan University, Shanghai, China.
*These authors contributed equally to this work.
Received 2024-10-11; Accepted 2025-1-18; Published 2025-2-10
Abstract
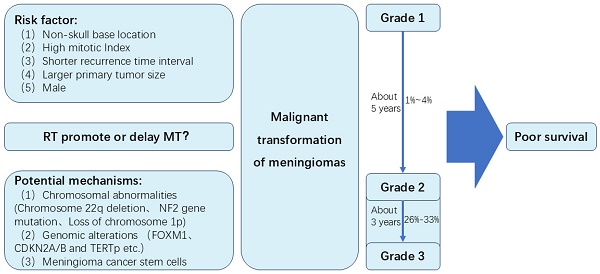
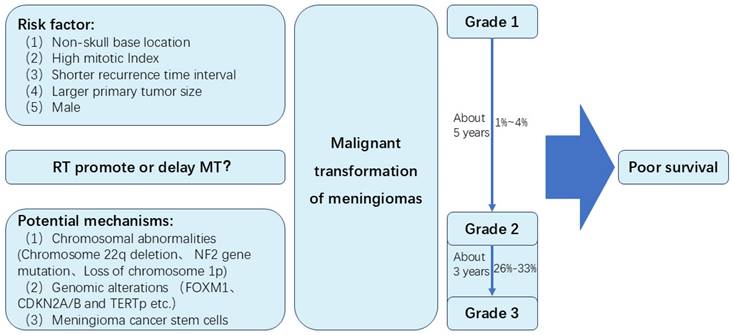
Meningioma is the most common intracranial tumor. Sometimes, meningiomas can develop malignant transformation (MT). In this review, we review the incidence of MT of meningiomas. The incidence of MT of grade 2 meningiomas is likely to be higher than benign meningiomas. Approximately 1% to 4% of WHO Grade 1 meningiomas may undergo MT, while about 26% to 33% of Grade 2 meningiomas experience MT. Time to MT of grade 2 meningiomas seemed to be shorter than MT of grade 1 meningiomas. The time for Grade I meningiomas to undergo MT is approximately 5 years, while Grade II meningiomas typically experience MT in about 3 years. Several risk factors may be associated with MT, including non-skull base location, high mitotic Index, a larger primary tumor size, shorter recurrence time interval and male. Potential molecular mechanisms of MT include chromosomal abnormalities (Chromosome 22q deletion, NF2 gene mutation, loss of chromosome 1p), genomic alterations (FOXM1, CDKN2A/B and TERTp), and meningioma cancer stem cells. Secondary meningiomas may have poor tumor control rates and overall survival rates than primary meningiomas. Besides, the role of radiotherapy in MT of meningiomas is unclear. Major concerns are whether radiotherapy can induce MT of meningiomas, and whether radiotherapy can prolong time to MT through long term control of meningiomas. This review summarizes the MT of meningiomas, and may provide the direction for further study of meningiomas.
Keywords: Meningioma, Malignant transformation, Secondary meningioma, Atypical meningioma, Malignant meningioma
Introduction
Meningiomas originate from meningothelial cells, are the most common primary intracranial tumors, and account for almost 39.0% and 54.5% of all and non-malignant central nervous system (CNS) tumors respectively [1]. According to WHO classification, meningiomas are divided into grade 1 (benign), 2 (atypical), and 3 (anaplastic). From the CBTRUS statistical report in the United States in 2014-2018, of meningioma with documented WHO grade (65.7%), 35.9%, 8.2% and 0.7% were WHO grade 1, 2 and 3 respectively. [1] Grade 1 meningiomas are usually relatively indolent and slowing tumors that display decelerated growth during tumor enlargement [2]. High grade meningiomas usually grow faster and are likely to recur.
The management of meningiomas includes surgical resection, radiotherapy, systematic therapy and observation. For most of symptomatic or enlarging meningiomas, surgery is the primary treatment [3]. Extent of resection (EOR) is a very important prognostic factor. However, event after gross total resection, meningiomas can recur. Approximately 20% of benign meningiomas are likely to be recurrent and invasive in fact [4]. Nearly 30%-40% of atypical meningiomas can recur [5]. Sometimes these recurrent lesions can transform to higher WHO grade, which is so-called malignant transformation (MT). In 1958, Hoffmann et al. [6] reported an autopsied case of a meningioma with MT and implantation in the subarachnoid space after periodic surgical removal over a period of 10 years. Since that, a small number of MT cases have been reported in many retrospective studies. However, many issues remain to be addressed, including the incidence, time to MT, risk factors, potential molecular mechanism and prognosis of MT. In this review, we will summarize some of the most recent advances of MT (Figure 1).
Summary of the malignant transformation of meningiomas in this study
Evolution of WHO Classification
Meningiomas have been classified according to WHO grading system since 1993 [7]. The WHO classification has been revised in 2000 [8], 2007 [9], 2016 [10] and 2021 [11]. In 2000, more specific criteria for grade 2 and 3 had been defined. Either of the following should be diagnosed as grade 2 meningiomas: (1) 4-19 mitoses per 10 HPF; (2) three or more of the following: increased cellularity, small cell change, prominent nucleoli, pattern less or sheet-like growth, foci of spontaneous or geographic necrosis [8]. And either of the following should be diagnosed as grade 3: (1) ≥20 mitoses per 10 HPF; (2) anaplastic (malignant) cytology resembling that of carcinoma, melanoma, or high-grade sarcoma [8]. In the revision of the 2007 WHO classification, predominant chordoid or clear cell morphology, and predominant papillary or rhabdoid morphology were added into the diagnosis of grade 2 and 3 respectively [9]. In 2016, brain invasion was added as a standalone diagnostic criterion for grade 2 meningiomas [10]. In 2021, molecular markers had been added as diagnostic criteria for selected subtypes. KLF4/TRAF7 mutations can be diagnosed as secretary meningiomas. SMARCE1 mutation was associated with clear cell meningiomas. Particularly, any meningioma with CDKN2A/B homozygous deletion or TERT promoter mutation should be diagnosed as WHO grade 3, regardless of histological criteria of anaplasia. Furthermore, papillary and rhabdoid subtypes were no longer allotted to grade 3 in absence of other criteria [11].
At present, there were some flaws in the molecular and histological diagnosis of meningiomas, including spatial and longitudinal heterogeneity of mutations, and subjective interpretation of histological criteria. These problems may be overcome by DNA methylation-based subtyping of meningioma, which may be superior to candidate gene panel sequencing and WHO classification for predicting time to tumor recurrence [12-15]. With growing knowledge of molecular diagnosis of meningioma, the WHO classification will continue to change. With each revision of WHO classification, the proportion of grade 2 and 3 meningiomas will have a little change.
Incidence of malignant transformation
Prior to the 2000 WHO classification, it had been reported almost 1-2% of grade 1 meningiomas transformed to high-grade meningiomas [16, 17]. Based on the 2000 WHO classification, Schiffer et al. [18] and McGovern et al. [19] reported nearly 4% of benign meningiomas progressed to grade 2 or 3 meningiomas. In the studies of Champeaux et al. [20] and Yeon et al. [21], 2.2% and 2.7% of grade 1 meningiomas exhibited MT according to the 2016 WHO classification respectively. It was unclear that whether these different proportion of MT were ascribed to the different version of WHO classification or the increasing use of radiotherapy. Therefore, in a recent systematic review and meta-analysis of MT of WHO grade 1 meningiomas, Nakasu et al. [22] reported 56 and 24 cases of MT were found in 2639 patients from surgery group and 5969 patients from radiosurgery group respectively. The incidence of MT was 2.98/1000 and 0.50/1000 patient-years in surgery and radiosurgery group respectively. However, due to a higher proportion of skull-base tumors and a lower proportion of reoperation for recurrent tumors, the incidence of MT was underestimated in radiosurgery group.
It was reported 26%-33% of atypical meningiomas occurred anaplastic transformation based on histological analysis at the time of recurrence [17]. Of those recurrent meningiomas, it was reported 6.3%-41.4% [16, 21, 23-25] could transform to higher grade tumors. During the history of malignant meningiomas, 14%-42.9% of cases were initially diagnosed as low-grade tumors [26]. As a higher recurrent rate and more aggressive behavior, the incidence of MT of grade 2 meningiomas is likely to be higher than benign meningiomas.
Time to malignant transformation
MT is a time-dependent event. In the study of Nakasu et al., [22] the median time to MT of WHO grade 1 meningiomas was 5 years (IQR, 2.5-8.2). Younger patients had a longer time to MT. For elderly patients (>50 years), time to MT was limited by life expectancy. The cumulative incidence curve of MT indicated a nearly linear increase in the first 8-9 years and a slower increase thereafter in the younger patients (≤50 years).
The median time of MT of grade 2 was about 3 years in most studies. [25-28] Kwon SM et al. [27] reported 9 cases of atypical meningiomas underwent MT to anaplastic meningiomas. The median time to MT was 19 months (range, 7-78). In the studies of Al-Mefty O et al. [25] and Yang et al. [28], the median time of grade 2 was 37 months (range, 12-82) and 39.8 months (range, 13.5-62.5) respectively. However, in these two studies, only 3 cases of MT patients were reported in each study. A relatively large number of MT cases was reported by Champeaux et al. [26], 50 cases of atypical meningioma underwent MT to anaplastic meningiomas with median time of 3.2 years (IQR, 1.2-4.9). The time to MT of grade 2 was approximately 3 years according to previous studies. It seemed to be shorter than MT of grade 1 meningiomas. This may be due to rapid tumor growth speed and short recurrent time in grade 2 meningiomas.
Risk factors associated with malignant transformation
Non-skull base location
Non-skull base meningiomas may be more prone to malignant transformation. In the study of Nakasu et al. [22], skull-base tumor location was significantly associated with MT of WHO grade 1 meningiomas, the higher proportion ofw skull-base location, the lower incidence of MT. McGovern et al. [19] revealed a similar result, patients with non-skull base meningiomas were likely to occur MT (36%) compared with patients with skull base meningiomas (5%, p=0.024). Due to a higher genomic instability and proliferative potential, [29-31] non-skull base meningiomas usually have different regrowth pattern and behavior. Skull base meningiomas often have a lower rate and plateau pattern of regrowth, while non-skull base meningiomas continue to grow [19, 29, 32]. Non-skull base meningiomas were significantly related with a higher WHO grade and a higher index of Ki67 [33]. Therefore, skull base meningiomas often have a relatively indolent nature history and a lower incident rate of MT.
High mitotic Index
The mitotic index is typically defined as the percentage of cells in mitosis (M-phase) within a specific tissue or cell population. This metric serves as a crucial indicator of aggressiveness and a high potential for proliferation [34-36]. Mitotic Index is one of the most important predictors of recurrence in meningiomas [37]. Kwon et al. [27, 38] conducted research to investigate the clinical factors that could predict the probability of MT of meningiomas. They found an increased mitotic index was the only significant predictor of MT of benign or atypical meningiomas. Nevertheless, variations in quantifying mitotic figures within 10 high-power fields (HPF) small unit areas arise due to subjective factors.
Shorter recurrence time interval and larger primary tumor size
After analyzing various clinical and radiological factors between the MT group and the non-MT group, researchers found that the recurrence interval for Grade 1 meningiomas that underwent MT was shorter than that of the non-MT group. Furthermore, when comparing the recurrence intervals of Grade 2 meningiomas, researchers discovered that the recurrence interval in the MT group was similar to that of Grade 2 meningiomas. These findings suggest that MT should be considered in patients with rapidly growing tumors following initial treatment [21]. Furthermore, a larger primary tumor size was found in high grade transformation group [21]. Tumor size had consistently been identified as a significant risk factor for recurrence [39, 40]. Larger tumors presented greater challenges for complete resection and may demonstrated a higher proliferative capacity of tumor cells. Given that meningioma progression results from multiple genetic mutations [25], the likelihood of genetic alterations leading to MT increases in highly cellular tumors.
Male
Meningiomas exhibit a higher prevalence in females compared to males. Prior epidemiological and pathological studies had demonstrated a link between female sex hormones and the propensity for meningioma formation [41-43]. Female/male ratio were 2.3:1 in non-malignant meningiomas [1], while male seemed to be a little predominance in grade 2 and 3 meningiomas [23, 44-47]. Some studies indicated a male predominance in the secondary meningiomas. Moliterno et al. [48] found a male predominance among the progressed group (64% VS 30%, p=0.04). Peyre et al. [49] and Krayenbuhl et al. [44] reported male patients prevailed in secondary anaplastic meningiomas. In the study of Sahm et al. [50] male was predominance in the TERT mutant cases (10/16) and associated with recurrence. However, in the study of Nakasu et al. [22] gender had no effect on the incidence rate of MT of grade 1 meningiomas in surgical series by meta-regression (p=0.088). Reports from the Nationwide Brain Tumor Registry of Japan indicated that male sex was associated with early recurrence of WHO grade I meningiomas after surgical resection. The gender-specific disparities in meningioma tumor behavior are presently not well-defined, necessitating additional examination of the underlying mechanisms. Due to the heightened prevalence of high-grade meningiomas in males and their potential connection to malignant transformation, special consideration may be warranted for male patients.
Potential molecular mechanisms of malignant transformation
Chromosomal abnormalities
Chromosome abnormality is one of the most important mechanisms of human malignant tumors, and has been an important part in progressive and recurrent meningiomas. Karyotypic abnormalities and copy-number alterations were associated with tumor aggressiveness [51, 52]. Chromosome 22q deletion is the most frequent chromosome abnormality in meningiomas. It was reported chromosome 22q occurred in more than half of meningiomas [53]. Deletion of chromosome 22q often occurred in the region of neurofibromatosis type 2 gene (NF2), leading to the occurrence of meningiomas [54]. Lots of studies revealed loss of gene function of NF2 was linked to the development of ependymomas, schwannoma and malignant mesothelioma [55-57]. Basic research suggested NF2 promoted contact inhibition and tumor suppression by inhibiting mitotic signaling in the cell cortex [58]. As a consequence, the inactivation of NF2 takes part in early oncogenic events. Genomic profiling revealed NF2 gene mutation was associated with chromosome instability and was an early and frequent event in MT meningioma samples [59]. However, the question was whether NF2 contributed to chromosome instability in meningiomas or whether NF2 loss was the consequence of an earlier event responsible for chromosome instability [59]. Some studies reported that the frequency of NF2 gene mutation was similar between benign and high grade meningiomas, indicating NF2 might not associated with progression of meningiomas [60].
Loss of chromosome 1p was associated with MT of meningiomas. Chromosome 1p harbored tumor suppressor genes associated with the malignant progression of meningiomas [61]. In malignant transformed meningiomas, 1p loss of heterozygosity and high methylation of the p73 promoter were detected, but were not detected in lower grade primary tumors [62]. The rate of deletion of chromosome 1p was increasing with WHO grade: 13%-26% in grade 1 meningiomas, 40%-76% in grade 2, 70%-100% in grade 3 [63]. Maas SLN et al. revealed the progression risk of WHO grade 1 meningiomas was significantly higher in cases exhibiting concurrent 1p/22q deletions (involving 6% or more of the chromosomal arms) than in cases without deletions or with only single 1p/22q deletions [64]. Analysis of chromosome 1p can provide an independent and cost-effective biomarker for identifying cases with a higher risk of recurrence [65].
Genomic alterations
Cell cycle dysregulation is associated with excessive tumor cell growth and proliferation, which could contribute to tumor recurrence and progression. Forkhead box protein M1 (FOXM1) is a master transcription factor for tumor cell growth and proliferation, which is related with several malignant tumors, including glioma, prostate cancer and hepatocellular carcinoma [66-68]. FOXM1 could promote mitotic progression by accelerating G1/S and G2/M transition and involve in meningioma progression [69, 70]. FOXM1 was associated with higher grade and recurrent meningiomas, and had shorter PFS [70]. FOXM1 was upregulated in premalignant grade 1 meningioma years before the grade 3 transformation [71]. Cyclin-dependent kinase inhibitor 2A/B (CDKN2A/B) encodes p16INK4A, p14ARF and p15INK4B. p16INK4A and p15INK4B could prevent S-phase entry by inhibiting the CDK4/cyclin D complex. p14ARF could prevent cell proliferation in G1 phase [72]. CDKN 2A/B locus loss on 9p was the most frequent recurrent genomic alterations progressing to grade 3 [59], and associated with poor prognosis in meningiomas [73]. Telomerase reverse transcriptase promoter (TERTp) mutations could promote cell immortalization and proliferation by preventing telomere shortening, and could enhance malignant behavior leading to poor prognosis [74, 75]. Researchers found acquisition of an activating TERTp mutation could lead to MT of meningiomas [76-78]. According to the literature, the incidence of TERT promoter mutations in meningioma patients with malignant histological progression was as high as 28% [79]. In addition, some patients exhibited histological progression, but TERT expression did not increase. This observation suggests that alternative mechanisms, such as alternative lengthening of telomeres, may be associated with telomere maintenance in meningiomas, similar to the situation observed in gliomas [80]. Some other gene mutations that are also associated with the MT of meningiomas, including TOP2A、BIRC5 and MYBL2 [71].
Meningioma cancer stem cells
Cancer stem cells (CSCs) represent a subpopulation within tumors that possess the ability for self-renewal and differentiation, functioning as crucial markers of tumor growth, metastasis, and treatment resistance [81]. Meningiomas are known to harbor CSCs, which are highly resilient and utilize deregulated stem cell expression profiles, thereby contributing to tumor recurrence, treatment resistance and MT [82-84]. The markers of CSCs in meningiomas include CD133, Sox2, nestin, and Frizzled-9 [85-88]. Baeesa SS et al. [84] reported a case of a Grade 1 meningioma that underwent MT into a Grade 3 meningioma, accompanied by extracranial metastasis. The authors observed that prior to the onset of metastasis, the tumor displayed CSC markers, the expression of which increased in the metastatic tissue. Additionally, primary cell lines derived from the metastatic tissue exhibited greater drug resistance and reduced apoptotic capacity. Consequently, because meningiomas can undergo MT, it may be feasible to predict this occurrence through the detection of CSCs.
Prognosis of malignant transformation
Recurrent meningiomas are biologically, clinically and pathologically more aggressive than primary meningiomas. Meningiomas with MT are usually recurrent tumors and undergo treatment failure, therefore, have similar characteristics as recurrent meningiomas. Previous studies had demonstrated with each successive tumor recurrence, the effectiveness of salvage treatment decreased [89, 90]. In the study of Momin et al. the median PFS after salvage radiotherapy for the 1st or 2nd recurrent grade 2 meningiomas was 47 months compared to 16 months for the 3rd or more recurrence (p=0.003) [90]. Prior radiotherapy also predicted poor prognosis. In the radiotherapy-naïve group, the 1-, 3- and 5-year PFS after salvage radiotherapy for recurrent grade 2 meningiomas were 96.3%, 65.8% and 44.2%, compared to 67.5%, 45.4% and 23.3% in the re-radiotherapy group (p=0.0084) [90]. Therefore, many studies had demonstrated secondary meningiomas had poor tumor control rates [28, 91-95] and overall survival rates [26, 28, 49, 92, 94] than primary meningiomas. While some studies found secondary meningiomas were not significantly associated with poor tumor control rates [89, 96-98] and overall survival rates [48, 95-97]. Table 1 summaries selected studies on prognosis of secondary meningiomas. In the secondary anaplastic meningiomas, Peyre et al. also found TERT mutations had a worse impact on the PFS [49]. Due to the limited number and small proportion of secondary meningiomas reported in the retrospective studies, the prognosis of secondary meningiomas need to be further investigated through high quality clinical researches.
Selected studies on prognosis of secondary meningiomas
| Study | Patients, n | IIary tumor, n(%) | WHO grade | Time to MT | Tumor control and risk factors | OS and risk factors | ||
|---|---|---|---|---|---|---|---|---|
| Wang et al. 2019 [91] | 263 | 31 (11.8) | 2 | NA | Median PFS: 2.3y | Tumor size≥41.5mm Extent of resection MIB-1>10 IIary meningiomas | NA | NA |
| Li et al. 2019 [92] | 302 | 52 (17.2) | 2 | Median: 3.2y | 5-y RFS: 47.7% | KPS≥80 Invasiveness IIary meningiomas | 5-y OS:78.8% | KPS≥80 PTE Supratentorial IIary meningiomas |
| Chen et al. 2018 [89] | 65 | 10 (15.4) | 2 | NA | 3-y LFFR:42% | Multifocal local recurrence | Median OS: 4.3y | NA |
| Champeaux et al. 2017 [93] | 215 | 18 (8.4) | 2 | Median: 5.7y | 5-y RFS: 82% | Simpson resection I/II Ki-67 IIary meningiomas | Median OS: 11.5y | NA |
| Champeaux et al. 2016 [94] | 194 | 31 (16.0) | 2 | Median: 5.7y | 5y-RFS: 71.6% | Simpson grade IIary meningiomas | 5y-OS: 83.2% | Age IIary meningiomas |
| Zhao et al. 2015 [95] | 89 | 11 (12.4) | 2 | NA | 5y-PFS: 67.5% | Symptom IIary meningiomas | 5y-OS: 89.1% | KPS score |
| Maier et al. 2022 [98] | 51 | 24 (47.1) | 3 | Median: 5.5 y | NA | NA | Median OS: 4.2 y | NA |
| Champeaux et al. 2019 [26] | 178 | 76 (42.7) | 3 | Medan: 7.5 y | NA | NA | 5-y OS: 40% | Age at MM surgery Completeness of resection Adjuvant RT IIary meningiomas |
| Peyre et al. 2018 [49] | 57 | 29 (50.8) | 3 | Median: 4.6 y | NA | NA | 5-y OS: 10% | Mitotic index IIary meningiomas |
| Zhao et al. 2015 [95] | 37 | 23 (62.2) | 3 | NA | 5-y PFS: 12.1% | IIary meningiomas | 5-y OS: 19.9% | Multi-occupation |
| Moliterno et al. 2015 [48] | 37 | 14 (37.8) | 3 | NA | NA | NA | 5-y OS: 27.9% | GTR at 1st surgery Convexity or parasagittal location |
| Yang et al. 2008 [28] | 74 | 20 (27.0) | 2/3 | Median: 5.8y (I-II); Median: 7.4y (I-III) Median: 3.3y (II-III) | 10-y DFS:87.1% (II); 5-y RFS:29% (III) | Brain invasion Adjuvant RT Extent of resection P53 expression IIary meningiomas | 10-y OS: 89.6% (II); 5-y OS:35% (III) | Brain invasion Adjuvant RT Extent of resection P53 expression IIary meningiomas |
| Pasquier et al. 2008 [96] | 119 | 16 (13.4) | 2/3 | Median 2.8±5y (I-II/III) | 5-y DFS: 58%; 10-y DFS:48% | High mitotic rate | 5-y OS: 65%; 10-y OS: 51% | Age > 60y High mitotic rate |
| Ferraro et al. 2014 [97] | 35 | 3 (8.6) | 2/3 | NA | 3-y PFS: 65% | Grade III tumors | 3-y OS: 78% | Grade III tumors |
Abbreviations: NA, not available; OS, overall survival, IQR, interquartile range; PFS, progression-free survival; RFS, recurrence-free survival; LFFR, local freedom from recurrence; DFS, disease free survival; PTE, peritumoral edema; MT, malignant transformation; IIary meningiomas, secondary meningiom
The relationship between radiotherapy and malignant transformation
Radiotherapy has been established as an alternative therapy to surgery, adjuvant or salvage treatment after surgical resection for meningiomas. A major concern is that whether radiotherapy can induce MT of meningiomas. Erroneous repair of DNA damage after radiotherapy can result in gross genomic rearrangement, which can lead to genomic instability, resistance to therapy, and tumorigenesis [99]. Meningiomas previously treated with adjuvant radiotherapy exhibit a significantly higher frequency of copy number alterations than radiation-naïve or radiation-induced meningiomas [100]. The implanted intracranial tumors formed by irradiated meningioma cell were found to be metastatic with secondary centers along the spinal cord, indicating higher aggressiveness, while the tumors formed by untreated meningioma cells were localized to the brain and did not show any morphological features, indicating less aggressive behavior [101]. These may be the mechanisms of MT induced by radiotherapy. In grade II IDH-mutant gliomas, chemotherapy and radiotherapy significantly increased malignant transformation rate per cell by 1.8 to 2.8 times compared with before treatment [102]. So far, more than one million patients have undergone stereotactic radiosurgery [103], rare individual case reports were associating stereotactic radiosurgery with MT into higher-grade meningiomas [104, 105] and other tumors [106-108]. It had been reported that radiotherapy can also induce the development of meningiomas. Meningiomas are the most common radiation-induced tumors after cranio-spinal radiotherapy, [109] with a 1/8 risk of developing radiation-induced meningiomas by the age of 40 [110-112]. Radiation-induced meningiomas were clinically aggressive, probably to be grade 2 meningiomas at first surgical resection (43.6%) and to progress after surgical resection (41%) [113]. Furthermore, second malignancies can be induced by previous radiotherapy. It was reported the 5- and 15-year probability to develop second tumors based on histopathology in or near the first radiotherapy area, after intermediate or high radiation doses, was 0.5% and 2.2%, respectively [114]. The overall median latency of second tumors was 7.4 years (1-42 years) [114]. These above studies support the view that radiotherapy can induce MT of meningiomas. However, even without radiotherapy, meningioma can develop MT similar to gliomas, when occur recurrence after surgical resection. A systematic review and meta-analysis [22] did not found evidence that radiosurgery increased the risk of MT of grade 1 meningiomas. These might be due to the grade 1 meningiomas treated with radiosurgery were more frequently located in the skull base, and less frequently treated with salvage surgery, leading to an unknown WHO classification at progression. It was difficult to compare the incidence rate of MT between surgery and radiotherapy group. Therefore, further study is needed to confirm the impact of radiotherapy on the incidence rate of malignant transformation.
Another major concern is that whether radiotherapy can prolong time to MT through long term control of meningiomas. Radiosurgery had demonstrated efficacy and safety for treating benign meningiomas even in the medium to long term [115]. It was reported WHO grade 1 meningiomas treated with radiosurgery had a long-term PFS ranging from 85% to 100% (median, 89%), and from 53% to 100% (median 85%) at 5 and 10 years respectively [116]. For postsurgical residual and recurrent WHO grade 2 meningiomas, radiotherapy is recommended [3]. Radiotherapy is a safe and effective treatment method for residual or recurrent grade 2 meningiomas. [117, 118] Sun et al. [117] reported SRS/EBRT had 2- and 5-year actuarial locoregional control rate of 91%/88% and 71%/69%, respectively. Aboukais et al. [118] also demonstrated the 1-, 2-, and 3-year actuarial local control rates and regional control rates for delayed progression after resection for grade 2 meningiomas were 75%, 52%, 40%, and 75%, 48%, 33%, respectively. Based on the efficacy of radiotherapy for meningiomas, it seems that time to MT could be prolonged. In IDH-mutant lower-grade gliomas (grade 2 or 3), radiotherapy is associated with delayed MT, the time to MT was 58.4 months in patients treated with radiotherapy compared with 32.6 months in patients without radiotherapy [119]. In the study of Nakasu et al., [22] time to MT was significantly longer in grade 1 meningiomas treated with radiotherapy before MT than in those who did not receive radiotherapy in univariate analyses, however, radiotherapy was not significantly associated with time to MT in multivariate analyses. These may be ascribed to high proportion of skull base meningiomas and low proportion of salvage surgery after radiotherapy in the patients who receive radiotherapy.
Conclusions
Meningioma is the most common tumor in CNS tumors. WHO classification has been widely used for predicting tumor recurrence. With the continuous deepening of research on meningioma and the revision of WHO classification every time, the proportion of WHO grade 2 and grade 3 changes dynamically. MT is one of the main reasons leading to treatment failure of meningiomas. In this review, we review the incidence of MT of meningiomas. Due to the higher recurrent rate and more aggressive behavior, the incidence of MT of grade 2 meningiomas is likely to be higher than benign meningiomas. Time to MT of grade 2 meningiomas seemed to be shorter than MT of grade 1 meningiomas. Several risk factors may be associated with MT, including non-skull base location, high mitotic Index, a larger primary tumor size, shorter recurrence time interval and male. Potential molecular mechanisms of MT include chromosomal abnormalities, genomic alterations. Secondary meningiomas are usually recurrent tumors and undergo treatment failure. Therefore, they may have poor tumor control rates and overall survival rates than primary meningiomas. Besides, the role of radiotherapy in MT of meningiomas is unclear. Major concerns are whether radiotherapy can induce MT of meningiomas, and whether radiotherapy can prolong time to MT through long term control of meningiomas. This review summarizes the MT of meningiomas, and may provide the direction for further study of meningiomas.
Acknowledgements
Funding
This study was supported by grants from the National Natural Science Foundation of China (82072788 to YG, 82203390 to LYH and 82203204 to JJD), the Science and Technology Commission of Shanghai Municipality (22140900200 to YG) and Shanghai Sailing Program (20YF1403900 to LYH).
Availability of data and materials
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
Jinxiu Yu and Jiaojiao Deng wrote the main manuscript and prepared table. Leihao Ren, Lingyang Hua, Ye Gong contributed to the main manuscript. Ye Gong and Lingyang Hua directed this work. All authors reviewed and edited the final manuscript.
Competing Interests
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
1. Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014-2018. Neuro-oncology. 2021;23:iii1-iii105
2. Nakasu S, Nakasu Y, Fukami T, Jito J, Nozaki K. Growth curve analysis of asymptomatic and symptomatic meningiomas. Journal of neuro-oncology. 2011;102:303-10
3. Goldbrunner R, Stavrinou P, Jenkinson MD, Sahm F, Mawrin C, Weber DC. et al. EANO guideline on the diagnosis and management of meningiomas. Neuro-oncology. 2021;23:1821-34
4. Perry A, Scheithauer BW, Stafford SL, Lohse CM, Wollan PC. "Malignancy" in meningiomas: a clinicopathologic study of 116 patients, with grading implications. Cancer. 1999;85:2046-56
5. Saraf S, McCarthy BJ, Villano JL. Update on meningiomas. Oncologist. 2011;16:1604-13
6. Hoffmann GT, Earle KM. Meningioma with malignant transformation and implantation in the subarachnoid space. Journal of neurosurgery. 1960;17:486-92
7. Kleihues P, Burger PC, Scheithauer BW. The new WHO classification of brain tumours. Brain Pathol. 1993;3:255-68
8. Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC. et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61:215-25 discussion 26-9
9. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A. et al. The 2007 WHO classification of tumours of the central nervous system. Acta neuropathologica. 2007;114:97-109
10. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK. et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta neuropathologica. 2016;131:803-20
11. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D. et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro-oncology. 2021;23:1231-51
12. Sahm F, Schrimpf D, Stichel D, Jones DTW, Hielscher T, Schefzyk S. et al. DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. The Lancet Oncology. 2017;18:682-94
13. Nassiri F, Mamatjan Y, Suppiah S, Badhiwala JH, Mansouri S, Karimi S. et al. DNA methylation profiling to predict recurrence risk in meningioma: development and validation of a nomogram to optimize clinical management. Neuro-oncology. 2019;21:901-10
14. Olar A, Wani KM, Wilson CD, Zadeh G, DeMonte F, Jones DT. et al. Global epigenetic profiling identifies methylation subgroups associated with recurrence-free survival in meningioma. Acta neuropathologica. 2017;133:431-44
15. Nassiri F, Liu J, Patil V, Mamatjan Y, Wang JZ, Hugh-White R. et al. A clinically applicable integrative molecular classification of meningiomas. Nature. 2021;597:119-25
16. Jellinger K, Slowik F. Histological subtypes and prognostic problems in meningiomas. Journal of neurology. 1975;208:279-98
17. Jaaskelainen J, Haltia M, Servo A. Atypical and anaplastic meningiomas: radiology, surgery, radiotherapy, and outcome. Surgical neurology. 1986;25:233-42
18. Schiffer D, Ghimenti C, Fiano V. Absence of histological signs of tumor progression in recurrences of completely resected meningiomas. Journal of neuro-oncology. 2005;73:125-30
19. McGovern SL, Aldape KD, Munsell MF, Mahajan A, DeMonte F, Woo SY. A comparison of World Health Organization tumor grades at recurrence in patients with non-skull base and skull base meningiomas. Journal of neurosurgery. 2010;112:925-33
20. Champeaux C, Houston D, Dunn L, Resche-Rigon M. Intracranial WHO grade I meningioma: a competing risk analysis of progression and disease-specific survival. Acta neurochirurgica. 2019;161:2541-9
21. Yeon EK, Sung JY, Do SI, Park BJ, Kim EJ, Na K. Clinicoradiological Features of Recurrent Meningioma With High Grade Transformation. Anticancer research. 2019;39:6299-305
22. Nakasu S, Notsu A, Na K, Nakasu Y. Malignant transformation of WHO grade I meningiomas after surgery or radiosurgery: systematic review and meta-analysis of observational studies. Neurooncol Adv. 2020;2:vdaa129
23. Rohringer M, Sutherland GR, Louw DF, Sima AA. Incidence and clinicopathological features of meningioma. Journal of neurosurgery. 1989;71:665-72
24. Violaris K, Katsarides V, Karakyriou M, Sakellariou P. Surgical Outcome of Treating Grades II and III Meningiomas: A Report of 32 Cases. Neurosci J. 2013;2013:706481
25. Al-Mefty O, Kadri PA, Pravdenkova S, Sawyer JR, Stangeby C, Husain M. Malignant progression in meningioma: documentation of a series and analysis of cytogenetic findings. Journal of neurosurgery. 2004;101:210-8
26. Champeaux C, Jecko V, Houston D, Thorne L, Dunn L, Fersht N. et al. Malignant Meningioma: An International Multicentre Retrospective Study. Neurosurgery. 2019;85:E461-E9
27. Kwon SM, Kim JH, Kim YH, Hong SH, Cho YH, Kim CJ. et al. Clinical Implications of the Mitotic Index as a Predictive Factor for Malignant Transformation of Atypical Meningiomas. Journal of Korean Neurosurgical Society. 2022;65:297-306
28. Yang SY, Park CK, Park SH, Kim DG, Chung YS, Jung HW. Atypical and anaplastic meningiomas: prognostic implications of clinicopathological features. Journal of neurology, neurosurgery, and psychiatry. 2008;79:574-80
29. Hashimoto N, Rabo CS, Okita Y, Kinoshita M, Kagawa N, Fujimoto Y. et al. Slower growth of skull base meningiomas compared with non-skull base meningiomas based on volumetric and biological studies. Journal of neurosurgery. 2012;116:574-80
30. Brastianos PK, Horowitz PM, Santagata S, Jones RT, McKenna A, Getz G. et al. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat Genet. 2013;45:285-9
31. Clark VE, Erson-Omay EZ, Serin A, Yin J, Cotney J, Ozduman K. et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science. 2013;339:1077-80
32. Mansouri A, Klironomos G, Taslimi S, Kilian A, Gentili F, Khan OH. et al. Surgically resected skull base meningiomas demonstrate a divergent postoperative recurrence pattern compared with non-skull base meningiomas. Journal of neurosurgery. 2016;125:431-40
33. Cornelius JF, Slotty PJ, Steiger HJ, Hanggi D, Polivka M, George B. Malignant potential of skull base versus non-skull base meningiomas: clinical series of 1,663 cases. Acta neurochirurgica. 2013;155:407-13
34. Kim D, Niemierko A, Hwang WL, Stemmer-Rachamimov AO, Curry WT, Barker FG. et al. Histopathological prognostic factors of recurrence following definitive therapy for atypical and malignant meningiomas. Journal of neurosurgery. 2018;128:1123-32
35. Perry A, Stafford SL, Scheithauer BW, Suman VJ, Lohse CM. Meningioma grading: an analysis of histologic parameters. Am J Surg Pathol. 1997;21:1455-65
36. Vranic A, Popovic M, Cor A, Prestor B, Pizem J. Mitotic count, brain invasion, and location are independent predictors of recurrence-free survival in primary atypical and malignant meningiomas: a study of 86 patients. Neurosurgery. 2010;67:1124-32
37. Olar A, Wani KM, Sulman EP, Mansouri A, Zadeh G, Wilson CD. et al. Mitotic Index is an Independent Predictor of Recurrence-Free Survival in Meningioma. Brain Pathol. 2015;25:266-75
38. Kwon SM, Kim JH, Yoo HJ, Kim YH, Hong SH, Cho YH. et al. Predictive factors for high-grade transformation in benign meningiomas. Clinical neurology and neurosurgery. 2020;195:105897
39. Anthofer J, Seidel-Schulz R, Proescholdt M, Brawanski A, Schebesch KM. Meningiomas Adjacent to Major Venous Sinuses-Clinical Outcome and Recurrence. World neurosurgery. 2017;104:560-6
40. Hortobagyi T, Bencze J, Varkoly G, Kouhsari MC, Klekner A. Meningioma recurrence. Open Med (Wars). 2016;11:168-73
41. Claus EB, Calvocoressi L, Bondy ML, Wrensch M, Wiemels JL, Schildkraut JM. Exogenous hormone use, reproductive factors, and risk of intracranial meningioma in females. Journal of neurosurgery. 2013;118:649-56
42. Khalid H. Immunohistochemical study of estrogen receptor-related antigen, progesterone and estrogen receptors in human intracranial meningiomas. Cancer. 1994;74:679-85
43. Pravdenkova S, Al-Mefty O, Sawyer J, Husain M. Progesterone and estrogen receptors: opposing prognostic indicators in meningiomas. Journal of neurosurgery. 2006;105:163-73
44. Krayenbuhl N, Pravdenkova S, Al-Mefty O. De novo versus transformed atypical and anaplastic meningiomas: comparisons of clinical course, cytogenetics, cytokinetics, and outcome. Neurosurgery. 2007;61:495-503 discussion -4
45. Gousias K, Schramm J, Simon M. The Simpson grading revisited: aggressive surgery and its place in modern meningioma management. Journal of neurosurgery. 2016;125:551-60
46. Kane AJ, Sughrue ME, Rutkowski MJ, Shangari G, Fang S, McDermott MW. et al. Anatomic location is a risk factor for atypical and malignant meningiomas. Cancer. 2011;117:1272-8
47. Mahmood A, Qureshi NH, Malik GM. Intracranial meningiomas: analysis of recurrence after surgical treatment. Acta neurochirurgica. 1994;126:53-8
48. Moliterno J, Cope WP, Vartanian ED, Reiner AS, Kellen R, Ogilvie SQ. et al. Survival in patients treated for anaplastic meningioma. Journal of neurosurgery. 2015;123:23-30
49. Peyre M, Gauchotte G, Giry M, Froehlich S, Pallud J, Graillon T. et al. De novo and secondary anaplastic meningiomas: a study of clinical and histomolecular prognostic factors. Neuro-oncology. 2018;20:1113-21
50. Sahm F, Schrimpf D, Olar A, Koelsche C, Reuss D, Bissel J. et al. TERT Promoter Mutations and Risk of Recurrence in Meningioma. Journal of the National Cancer Institute. 2016;108:djv377
51. Cordova C, Kurz SC. Advances in Molecular Classification and Therapeutic Opportunities in Meningiomas. Current oncology reports. 2020;22:84
52. Aizer AA, Abedalthagafi M, Bi WL, Horvath MC, Arvold ND, Al-Mefty O. et al. A prognostic cytogenetic scoring system to guide the adjuvant management of patients with atypical meningioma. Neuro-oncology. 2016;18:269-74
53. Tang M, Wei H, Han L, Deng J, Wang Y, Yang M. et al. Whole-genome sequencing identifies new genetic alterations in meningiomas. Oncotarget. 2017;8:17070-80
54. Leone PE, Bello MJ, de Campos JM, Vaquero J, Sarasa JL, Pestana A. et al. NF2 gene mutations and allelic status of 1p, 14q and 22q in sporadic meningiomas. Oncogene. 1999;18:2231-9
55. Lee CH, Chung CK, Ohn JH, Kim CH. The Similarities and Differences between Intracranial and Spinal Ependymomas: A Review from a Genetic Research Perspective. Journal of Korean Neurosurgical Society. 2016;59:83-90
56. Quetel L, Meiller C, Assie JB, Blum Y, Imbeaud S, Montagne F. et al. Genetic alterations of malignant pleural mesothelioma: association with tumor heterogeneity and overall survival. Mol Oncol. 2020;14:1207-23
57. Yao L, Alahmari M, Temel Y, Hovinga K. Therapy of Sporadic and NF2-Related Vestibular Schwannoma. Cancers. 2020;12:835
58. McClatchey AI, Fehon RG. Merlin and the ERM proteins-regulators of receptor distribution and signaling at the cell cortex. Trends Cell Biol. 2009;19:198-206
59. Goutagny S, Yang HW, Zucman-Rossi J, Chan J, Dreyfuss JM, Park PJ. et al. Genomic profiling reveals alternative genetic pathways of meningioma malignant progression dependent on the underlying NF2 status. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16:4155-64
60. Riemenschneider MJ, Perry A, Reifenberger G. Histological classification and molecular genetics of meningiomas. The Lancet Neurology. 2006;5:1045-54
61. Ishino S, Hashimoto N, Fushiki S, Date K, Mori T, Fujimoto M. et al. Loss of material from chromosome arm 1p during malignant progression of meningioma revealed by fluorescent in situ hybridization. Cancer. 1998;83:360-6
62. Nakane Y, Natsume A, Wakabayashi T, Oi S, Ito M, Inao S. et al. Malignant transformation-related genes in meningiomas: allelic loss on 1p36 and methylation status of p73 and RASSF1A. Journal of neurosurgery. 2007;107:398-404
63. Lamszus K. Meningioma pathology, genetics, and biology. J Neuropathol Exp Neurol. 2004;63:275-86
64. Maas SLN, Hielscher T, Sievers P, Hovestadt V, Suwala AK, Acker T. et al. Loss over 5% of chromosome 1p is a clinically relevant and applicable cut-off for increased risk of recurrence in meningioma. Acta neuropathologica. 2024;148:17
65. Maas SLN, Sievers P, Weber DC, Weller M, van den Bent MJ, Mair MJ. et al. Independent prognostic impact of DNA methylation class and chromosome 1p loss in WHO grade 2 and 3 meningioma undergoing adjuvant high-dose radiotherapy: comprehensive molecular analysis of EORTC 22042-26042. Acta neuropathologica. 2023;146:837-40
66. Kalinichenko VV, Major ML, Wang X, Petrovic V, Kuechle J, Yoder HM. et al. Foxm1b transcription factor is essential for development of hepatocellular carcinomas and is negatively regulated by the p19ARF tumor suppressor. Genes Dev. 2004;18:830-50
67. Kalin TV, Wang IC, Ackerson TJ, Major ML, Detrisac CJ, Kalinichenko VV. et al. Increased levels of the FoxM1 transcription factor accelerate development and progression of prostate carcinomas in both TRAMP and LADY transgenic mice. Cancer Res. 2006;66:1712-20
68. Liu M, Dai B, Kang SH, Ban K, Huang FJ, Lang FF. et al. FoxM1B is overexpressed in human glioblastomas and critically regulates the tumorigenicity of glioma cells. Cancer Res. 2006;66:3593-602
69. Alvarez-Fernandez M, Medema RH. Novel functions of FoxM1: from molecular mechanisms to cancer therapy. Frontiers in oncology. 2013;3:30
70. Kim H, Park KJ, Ryu BK, Park DH, Kong DS, Chong K. et al. Forkhead box M1 (FOXM1) transcription factor is a key oncogenic driver of aggressive human meningioma progression. Neuropathol Appl Neurobiol. 2020;46:125-41
71. Maier AD, Meddis A, Mirian C, Haslund-Vinding J, Bartek J, Krog SM. et al. Gene expression analysis during progression of malignant meningioma compared to benign meningioma. Journal of neurosurgery. 2023;138:1302-12
72. Bostrom J, Meyer-Puttlitz B, Wolter M, Blaschke B, Weber RG, Lichter P. et al. Alterations of the tumor suppressor genes CDKN2A (p16(INK4a)), p14(ARF), CDKN2B (p15(INK4b)), and CDKN2C (p18(INK4c)) in atypical and anaplastic meningiomas. Am J Pathol. 2001;159:661-9
73. Sievers P, Hielscher T, Schrimpf D, Stichel D, Reuss DE, Berghoff AS. et al. CDKN2A/B homozygous deletion is associated with early recurrence in meningiomas. Acta neuropathologica. 2020;140:409-13
74. Juratli TA, Thiede C, Koerner MVA, Tummala SS, Daubner D, Shankar GM. et al. Intratumoral heterogeneity and TERT promoter mutations in progressive/higher-grade meningiomas. Oncotarget. 2017;8:109228-37
75. Spiegl-Kreinecker S, Lotsch D, Neumayer K, Kastler L, Gojo J, Pirker C. et al. TERT promoter mutations are associated with poor prognosis and cell immortalization in meningioma. Neuro-oncology. 2018;20:1584-93
76. Harmanci AS, Youngblood MW, Clark VE, Coskun S, Henegariu O, Duran D. et al. Integrated genomic analyses of de novo pathways underlying atypical meningiomas. Nat Commun. 2018;9:16215
77. Hong Y, Han N, Gwak HS. Malignant Transformation of Meningioma With TERT Promoter Mutation: A Case Report. Brain tumor research and treatment. 2024;12:192-9
78. Maier AD, Stenman A, Svahn F, Mirian C, Bartek J Jr, Juhler M. et al. TERT promoter mutations in primary and secondary WHO grade III meningioma. Brain Pathol. 2021;31:61-9
79. Goutagny S, Nault JC, Mallet M, Henin D, Rossi JZ, Kalamarides M. High incidence of activating TERT promoter mutations in meningiomas undergoing malignant progression. Brain Pathol. 2014;24:184-9
80. Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR. et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462-77
81. Chang JC. Cancer stem cells: Role in tumor growth, recurrence, metastasis, and treatment resistance. Medicine. 2016;95:S20-S5
82. Shivapathasundram G, Wickremesekera AC, Tan ST, Itinteang T. Tumour stem cells in meningioma: A review. Journal of clinical neuroscience: official journal of the Neurosurgical Society of Australasia. 2018;47:66-71
83. Freitag D, McLean AL, Simon M, Koch A, Grube S, Walter J. et al. NANOG overexpression and its correlation with stem cell and differentiation markers in meningiomas of different WHO grades. Mol Carcinog. 2017;56:1953-64
84. Baeesa SS, Hussein D, Altalhy A, Bakhaidar MG, Alghamdi FA, Bangash M. et al. Malignant Transformation and Spine Metastasis of an Intracranial Grade I Meningioma: In Situ Immunofluorescence Analysis of Cancer Stem Cells Case Report and Literature Review. World neurosurgery. 2018;120:274-89
85. Tang H, Gong Y, Mao Y, Xie Q, Zheng M, Wang D. et al. CD133-positive cells might be responsible for efficient proliferation of human meningioma cells. International journal of molecular sciences. 2012;13:6424-39
86. Hueng DY, Sytwu HK, Huang SM, Chang C, Ma HI. Isolation and characterization of tumor stem-like cells from human meningiomas. Journal of neuro-oncology. 2011;104:45-53
87. Khan I, Baeesa S, Bangash M, Schulten HJ, Alghamdi F, Qashqari H. et al. Pleomorphism and drug resistant cancer stem cells are characteristic of aggressive primary meningioma cell lines. Cancer Cell Int. 2017;17:72
88. Mimeault M, Batra SK. Molecular biomarkers of cancer stem/progenitor cells associated with progression, metastases, and treatment resistance of aggressive cancers. Cancer Epidemiol Biomarkers Prev. 2014;23:234-54
89. Chen WC, Hara J, Magill ST, Wu A, Aghi MK, Theodosopoulos PV. et al. Salvage therapy outcomes for atypical meningioma. Journal of neuro-oncology. 2018;138:425-33
90. Momin AA, Shao J, Soni P, Almeida JP, Suh JH, Murphy ES. et al. Outcomes of salvage radiation for recurrent world health organization grade II meningiomas: a retrospective cohort study. Journal of neuro-oncology. 2021;152:373-82
91. Wang F, Xu D, Liu Y, Lin Y, Wei Q, Gao Q. et al. Risk factors associated with postoperative recurrence in atypical intracranial meningioma: analysis of 263 cases at a single neurosurgical centre. Acta neurochirurgica. 2019;161:2563-70
92. Li H, Zhang YS, Zhang GB, Zhang GJ, Wang B, Li D. et al. Treatment Protocol, Long-Term Follow-Up, and Predictors of Mortality in 302 Cases of Atypical Meningioma. World neurosurgery. 2019;122:e1275-e84
93. Champeaux C, Houston D, Dunn L. Atypical meningioma. A study on recurrence and disease-specific survival. Neurochirurgie. 2017;63:273-81
94. Champeaux C, Wilson E, Shieff C, Khan AA, Thorne L. WHO grade II meningioma: a retrospective study for outcome and prognostic factor assessment. Journal of neuro-oncology. 2016;129:337-45
95. Zhao P, Hu M, Zhao M, Ren X, Jiang Z. Prognostic factors for patients with atypical or malignant meningiomas treated at a single center. Neurosurgical review. 2015;38:101-7 discussion 7
96. Pasquier D, Bijmolt S, Veninga T, Rezvoy N, Villa S, Krengli M. et al. Atypical and malignant meningioma: outcome and prognostic factors in 119 irradiated patients. A multicenter, retrospective study of the Rare Cancer Network. International journal of radiation oncology, biology, physics. 2008;71:1388-93
97. Ferraro DJ, Funk RK, Blackett JW, Ju MR, DeWees TA, Chicoine MR. et al. A retrospective analysis of survival and prognostic factors after stereotactic radiosurgery for aggressive meningiomas. Radiation oncology (London, England). 2014;9:38
98. Maier AD, Mirian C, Haslund-Vinding J, Bartek J, Guldager R, Moller S. et al. Granular clinical history and outcome in 51 patients with primary and secondary malignant meningioma. Journal of neurosurgery. 2022;137:1347-57
99. Santivasi WL, Xia F. Ionizing radiation-induced DNA damage, response, and repair. Antioxid Redox Signal. 2014;21:251-9
100. Bi WL, Greenwald NF, Abedalthagafi M, Wala J, Gibson WJ, Agarwalla PK. et al. Genomic landscape of high-grade meningiomas. NPJ Genom Med. 2017;2:2-15
101. Gogineni VR, Nalla AK, Gupta R, Dinh DH, Klopfenstein JD, Rao JS. Chk2-mediated G2/M cell cycle arrest maintains radiation resistance in malignant meningioma cells. Cancer Lett. 2011;313:64-75
102. Aoki K, Suzuki H, Yamamoto T, Yamamoto KN, Maeda S, Okuno Y. et al. Mathematical Modeling and Mutational Analysis Reveal Optimal Therapy to Prevent Malignant Transformation in Grade II IDH-Mutant Gliomas. Cancer Res. 2021;81:4861-73
103. Pollock BE, Link MJ, Stafford SL, Parney IF, Garces YI, Foote RL. The Risk of Radiation-Induced Tumors or Malignant Transformation After Single-Fraction Intracranial Radiosurgery: Results Based on a 25-Year Experience. International journal of radiation oncology, biology, physics. 2017;97:919-23
104. Mattozo CA, De Salles AA, Klement IA, Gorgulho A, McArthur D, Ford JM. et al. Stereotactic radiation treatment for recurrent nonbenign meningiomas. Journal of neurosurgery. 2007;106:846-54
105. Starke RM, Williams BJ, Hiles C, Nguyen JH, Elsharkawy MY, Sheehan JP. Gamma knife surgery for skull base meningiomas. Journal of neurosurgery. 2012;116:588-97
106. Osipov V, Ho KC, Krouwer HG, Meyer G, Shidham VB. Post-radiation dedifferentiation of meningioma into osteosarcoma. BMC Cancer. 2002;2:34
107. Lee HS, Kim JH, Lee JI. Glioblastoma following radiosurgery for meningioma. Journal of Korean Neurosurgical Society. 2012;51:98-101
108. Lall RR, Lall RR, Smith TR, Lee KH, Mao Q, Kalapurakal JA. et al. Delayed malignant transformation of petroclival meningioma to chondrosarcoma after stereotactic radiosurgery. Journal of clinical neuroscience: official journal of the Neurosurgical Society of Australasia. 2014;21:1225-8
109. Galloway TJ, Indelicato DJ, Amdur RJ, Swanson EL, Morris CG, Marcus RB. Favorable outcomes of pediatric patients treated with radiotherapy to the central nervous system who develop radiation-induced meningiomas. International journal of radiation oncology, biology, physics. 2011;79:117-20
110. Vinchon M, Leblond P, Caron S, Delestret I, Baroncini M, Coche B. Radiation-induced tumors in children irradiated for brain tumor: a longitudinal study. Child's nervous system: ChNS: official journal of the International Society for Pediatric Neurosurgery. 2011;27:445-53
111. Kok JL, Teepen JC, van Leeuwen FE, Tissing WJE, Neggers S, van der Pal HJ. et al. Risk of benign meningioma after childhood cancer in the DCOG-LATER cohort: contributions of radiation dose, exposed cranial volume, and age. Neuro-oncology. 2019;21:392-403
112. Remes TM, Suo-Palosaari MH, Heikkila VP, Sutela AK, Koskenkorva PKT, Toiviainen-Salo SM. et al. Radiation-Induced Meningiomas After Childhood Brain Tumor: A Magnetic Resonance Imaging Screening Study. J Adolesc Young Adult Oncol. 2019;8:593-601
113. Gillespie CS, Islim AI, Taweel BA, Millward CP, Kumar S, Rathi N. et al. The growth rate and clinical outcomes of radiation induced meningioma undergoing treatment or active monitoring. Journal of neuro-oncology. 2021;153:239-49
114. Welte B, Suhr P, Bottke D, Bartkowiak D, Dorr W, Trott KR. et al. Second malignancies in high-dose areas of previous tumor radiotherapy. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft [et al]. 2010;186:174-9
115. Santacroce A, Walier M, Regis J, Liscak R, Motti E, Lindquist C. et al. Long-term tumor control of benign intracranial meningiomas after radiosurgery in a series of 4565 patients. Neurosurgery. 2012;70:32-9 discussion 9
116. Marchetti M, Sahgal A, De Salles AAF, Levivier M, Ma L, Paddick I. et al. Stereotactic Radiosurgery for Intracranial Noncavernous Sinus Benign Meningioma: International Stereotactic Radiosurgery Society Systematic Review, Meta-Analysis and Practice Guideline. Neurosurgery. 2020;87:879-90
117. Sun SQ, Cai C, Murphy RK, DeWees T, Dacey RG, Grubb RL. et al. Radiation Therapy for Residual or Recurrent Atypical Meningioma: The Effects of Modality, Timing, and Tumor Pathology on Long-Term Outcomes. Neurosurgery. 2016;79:23-32
118. Aboukais R, Zairi F, Lejeune JP, Le Rhun E, Vermandel M, Blond S. et al. Grade 2 meningioma and radiosurgery. Journal of neurosurgery. 2015;122:1157-62
119. Liu Y, Chen H, Li G, Zhang J, Yao K, Wu C. et al. Radiotherapy delays malignant transformation and prolongs survival in patients with IDH-mutant gliomas. Cancer Biol Med. 2022;19:1477-86
Author contact
![]() Corresponding authors: Lingyang Hua, MD, PhD, Department of Neurosurgery, Huashan Hospital, Shanghai Medical College, Fudan University, No 12 Middle Wulumuqi Rd, Jingan District, Shanghai 200040, China. Email: hua.ling.yangcom; Ye Gong, MD, Department of Neurosurgery and Department of Critical Care Medicine, Huashan Hospital, Shanghai Medical College, Fudan University, No 12 Middle Wulumuqi Rd, Jingan District, Shanghai 200040, China. Email: drgongyecom.
Corresponding authors: Lingyang Hua, MD, PhD, Department of Neurosurgery, Huashan Hospital, Shanghai Medical College, Fudan University, No 12 Middle Wulumuqi Rd, Jingan District, Shanghai 200040, China. Email: hua.ling.yangcom; Ye Gong, MD, Department of Neurosurgery and Department of Critical Care Medicine, Huashan Hospital, Shanghai Medical College, Fudan University, No 12 Middle Wulumuqi Rd, Jingan District, Shanghai 200040, China. Email: drgongyecom.

 Global reach, higher impact
Global reach, higher impact