Impact Factor
ISSN: 1837-9664
J Cancer 2018; 9(23):4527-4535. doi:10.7150/jca.28532 This issue Cite
Research Paper
Oxymatrine Synergistically Potentiates the Antitumor Effects of Cisplatin in Human Gastric Cancer Cells
1. Department of General Surgery, Wujiang No.1 People's Hospital affiliated to Nantong University, Suzhou, Jiangsu 215200, PR China
2. Department of General Surgery, Hepatobiliary surgery, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu 215200, PR China
*These authors contributed equally to this work
Received 2018-7-15; Accepted 2018-10-3; Published 2018-10-31
Abstract
Cisplatin (CDDP) has been extensively used for gastric cancer (GC) treatment but limited by drug resistance and severe toxicity. The chemo-sensitizers that enhance its efficiency and overcome its limitation are urgently needed. Oxymatrine (OMT), a primary active ingredient from the dry roots of Sophora favescens, has shown powerful anti-cancer property with little side-effect. In this study, we explored the chemo-sensitization of OMT to potentiate the anti-tumor effect of CDDP. GC cell lines were dealt with OMT and/or CDDP and then subjected to different experimental methods. We found that OMT could significantly potentiate the CDDP-caused BGC-823 and SGC7901 cells viability loss, and OMT acts synergistically with CDDP. The combinative treatment could arrest cell cycle in G0/G1 phase by increasing p21, p27 and decreasing cyclin D1, and induced apoptosis by ROS generation and AKT/ERK inactivation. Inhibition of ROS respectively reversed the cell death induced by OMT and/or CDDP, suggesting the pivotal roles of ROS in the process. Moreover, OMT enhanced the antitumor effects of CDDP in nude mice bearing BGC823 tumor xenografts in vivo. Taken together, this study highlights that the co-treatment with OMT and CDDP exerted synergistic antitumor effects in GC cells, and that these effects may be mediated by ROS generation and inactivation of the AKT/ERK pathways.
Keywords: Oxymatrine, Gastric cancer, Cisplatin, Synergistically, Reactive oxygen species
Introduction
Gastric cancer (GC) is one of the most common cancers and the second leading cause of cancer deaths worldwide [1]. GC is a serious threat to humans because of its high incidence and mortality [2], and China got the highest incidence of GC [3]. Despite great advances made in surgical techniques and chemotherapeutic agents, the 5-year survival rate of patients with GC is still dissatisfaction due to lack of early diagnosis and chemo-resistance [4, 5]. Therefore, new and safe strategies to overcome chemotherapeutic resistance and minimize side effects are under exploration.
Cisplatin (CDDP) is one of the most frequently used chemotherapeutic drugs for GC treatment [6]. Recently, a study reported that the combination of CDDP and S-1 (the 5-fluorouracil-related drug) could be considered the first-line chemotherapy treatment for advanced GC [7]. However, the therapeutic efficacy of CDDP treatment to GC patients is limited since the emergence of drug resistance [8]. Thus, it's necessary to identify novel compounds that can be used to enhance the response of GC cells to chemotherapeutic agents.
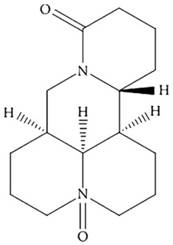
Oxymatrine (OMT; Figure 1) is a primary active ingredient from the dry roots of Sophora favescens [9]. It has been widely studied in various types of malignancies, including GC [10-13]. We also found that OMT could inhibit human hepatocellular carcinoma cell growth [14] and synergistically potentiates antitumor activity of oxaliplatin in colon carcinoma through PI3K/AKT/mTOR pathway [15]. However, the effect of OMT combined with CDDP on the growth of GC cells and the underlying molecular mechanisms remain unknown.
Thus, in this study, the important roles of OMT and CDDP in regulating GC cells growth, apoptosis, cycle and invasion were investigated. And we found that OMT synergistically enhanced the anti-tumor efficacy of CDDP against GC cells. These findings demonstrated that OMT alone or combined with CDDP is promising in treating GC.
The chemical structure of OMT.
Materials and Methods
Cell culture and reagents
Human GC SGC7901 and BGC823 cells were purchased from the Shanghai Cell Institute Country Cell Bank (Shanghai, China) and cultured in RPMI-1640 medium (Gibco, Gaithersburg, MD, USA), then incubated at 37°C in 5% CO2. OMT was purchased from Nanjing Zelang Medical Technology Co, Ltd (Number: ZL10603GZ, Jiangsu, China), CDDP was purchased from Selleck Chemicals (Houston, TX, USA).
Cell viability and clone formation assays
The MTT assay (Sigma Chemical Company, St. Louis, California) was used to detect cell viability. Cells were trypsinized and seeded in 96-well plates (1×104 cells/well) and cultured overnight, then dealt with various concentrations of CDDP and OMT respectively and concurrently. After treatment for 48h, MTT (5mg/ml) were added and incubated for another 4h. After removing the supernatant, 200μl dimethyl sulfoxide (DMSO, Sigma, USA) were added. The absorbance values at 490nm were measured. The proliferative inhibition rate was calculated as follows: (1-experimental group/control group) × 100%.
For colony formation analysis, BGC823 cells (1000cells/well) were added into 6-well plates and dealt with OMT, CDDP, or OMT + CDDP for 12h. After this, the medium was replaced and the cells were cultured for an additional 10 days. Colonies were fixed and stained using Giemsa (Sigma Chemical Company, St. Louis, California) for 30min, then counted in Image J software (National Institutes of Health) [16].
Synergy determination
The data obtained from the cell viability assay were standardized to the control group and showed as % viability. Further, the combination index (CI) was calculated according to Chou's CI model using CalcuSyn software program [17]. The CI values represent the modes of the interaction between two drugs. CI = 1 means additive effect, CI < 1 means synergistic effect, and CI > 1 means antagonistic effect.
Hoechst staining
BGC823 cells were stained by Hoechst 33342 in the dark for 15min after treated for 48h, then washed with phosphate-buffered saline (PBS) and photographed under a fluorescence microscope. The apoptosis index of cells was calculated as follows: apoptotic cells/total cells × 100%.
Cell apoptosis and cycle analysis
BGC823 cells were respectively treated with OMT and/or CDDP for 48h, then harvested and conducted to flow cytometry analysis (Becton Dickinson, Franklin Lakes, NJ, USA) [18]. For cell cycle analysis, after the indicated treatments, BGC823 cells were analyzed by Becton Dickinson FACScan flow cytometer in the presence of PI buffer with RNase (Beyotime Institute, Jiangsu, China).
Transwell assay
Cell invasion capacity of BGC823 cells was detected by transwell chamber assay (Millipore, Billerica, MA, USA). BGC823 cells (4×104 cells) were suspension in 200μL serum-free medium containing different drug treatments and cultured on the above compartment, and 500μL full medium including 10% fatal bovine serum (FBS) was added to the nether compartment. The cells on the upper chamber were discarded after 36h; the cells on the lower surface of the membrane were stained with crystal violet and then photographed and counted using the microscope.
Reactive oxygen species (ROS) assay
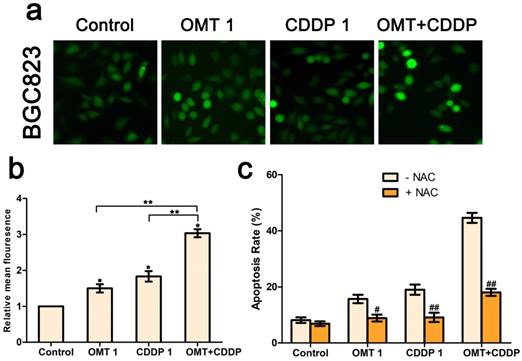
ROS levels were probed using DCFH-DA (Molecular Probes Inc., Eugene, OR). BGC823 cells were exposed to OMT, CDDP or OMT + CDDP for 24h and then washed with phosphate-buffered saline (PBS) for three times. After that, cells were treated with DCFH-DA for 30min and viewed under a fluorescence microscope (Nikon, Tokyo, Japan). Besides, the apoptosis of BGC823 cells was analyzed again after NAC (5mM; Sigam-Aldrich, St. Louis, MO) was used as a ROS scavenger added to culture medium simultaneously with CDDP and/or OMT adding.
Western blot analysis
Briefly, BGC823 cells were dealt with different drugs for 36h and then lysed with lysis bufer (Beyotime Institute of Biotechnology, Shanghai, China). All the selected protein extracts were separated by sodium dodecylsulphate polyacrylamide gel electrophoresis (SDS-PAGE) and transblotted to a polyvinylidene difluoridex (PVDF) membrane (Beyotime Institute of Biotechnology). The membranes were blocked with 5% fresh nonfat milk dissolved in tris buffer solution tween (TBST) and incubated with primary and secondary antibodies, respectively. Signals were visualized using an ECL kit (Bio-Rad, Hercules, CA). The bands were semiquantified using Image J software. AKT, p-AKT, ERK, p-ERK, cyclinD1, p21 and p27 antibodies were purchased from Cell Signaling Technology (MA, USA), and the GAPDH antibody was from Kangchen Bio-tech (Shanghai, China).
Tumor xenograft study
Male 4-5 weeks-old BALB/c nude mice (Shanghai SLAC Laboratory Animal Center, Shanghai, China) were used to establish GC xenograft model. BGC823 cells (2×106/100μl) were implanted by subcutaneous injected into the right axillary fossa of mouse. After one week, the animals were randomly divided into four groups (six mice per group) and given: (1) control (sterile physiological saline, i.p., every other day), (2) OMT (30mg/kg, i.p., every other day), (3) CDDP (5mg/kg, i.p., every other day), (4) OMT + CDDP (30mg/kg + 5mg/kg, i.p., every other day). After 24 days, mice were sacrificed and xenografts tumors were harvested and weighed. The tumor volume and inhibition ratio were tested as described before [19].
The paraffin-embedded sections (5μm thick) were prepared and stained with hematoxylin and eosin (H&E) or immunohistochemically with p-AKT, p-ERK or Ki-67 (mouse anti-Ki67 antibody; GeneTex Inc, Irvine, California) according to previously reported protocols [19, 20].
Statistical analysis
Data were shown as means and standard deviation (SD) values and performed using the SPSS 17.0 software (SPSSInc, Chicago). Comparisons among multiple groups were assessed using one-way ANOVA and the p-value of less than 0.05 indicates statistically significant.
Results
OMT enhanced the inhibitory effects of CDDP on the viability of the GC cells
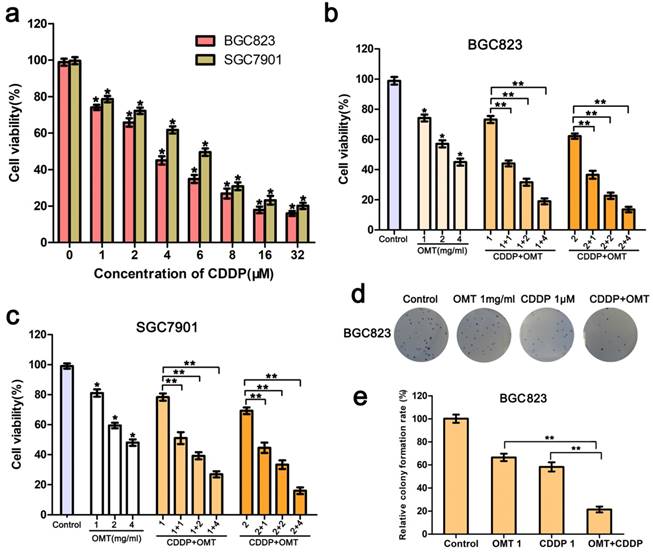
A dose-response study was performed with OMT and CDDP in BGC823 and SGC7901 cells. As shown in Fig 2a, The IC50 value (half maximal inhibitory concentration) was calculated as 3.36 ± 0.68μM for BGC823 and 6.08 ± 1.22μM for SGC7901 cells; BGC823 cells performed more sensitive than SGC7901 cells to CDDP treatment. The growth of BGC823 and SGC7901 cells were inhibited in a dose-dependent manner by OMT. When cells were treated with CDDP (1, 2μM) and OMT (1.0, 2.0, 4.0 mg/ml) simultaneously, the combined treatment showed a stronger inhibitory effect on cell growth than either drug alone (Fig 2b-c).
Next, we calculated the CI using the CompuSyn software and the Chou-Talalay method. The CI value for CDDP (1μM) combined with OMT (1mg/ml) was 0.61 ± 0.08 for BGC823 cells and 0.75 ± 0.11 for SGC7901 cells. For BGC823 cells, the combination of CDDP (1μM) and OMT (1mg/ml) showed the best synergistic inhibition capacity, which was used in all subsequent experiments.
Furthermore, as show in Fig 2d-e, We found that the colony number and size obviously decreased after treatment with OMT or CDDP, and significantly fewer colonies were observed in the OMT plus CDDP treatment group.
OMT synergistically enhanced CDDP-induced apoptosis in GC cells
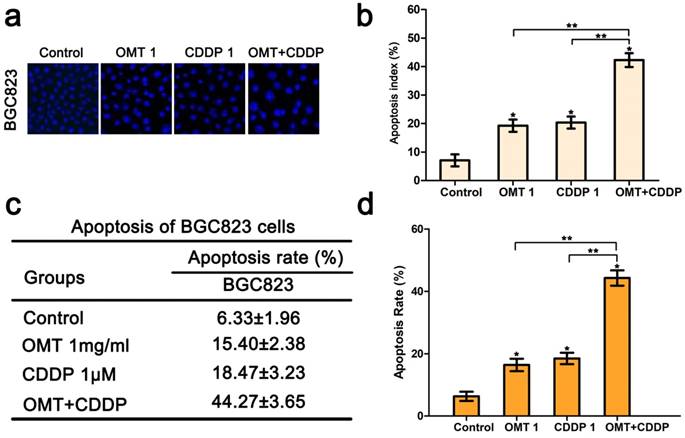
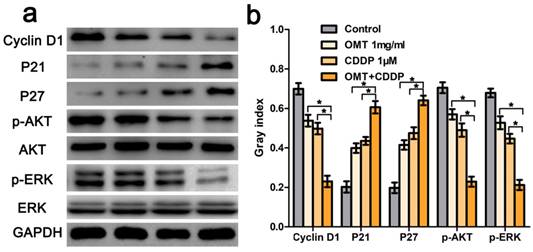
Hoechst33342 staining demonstrated that morphological changes were found in cells treated with OMT or CDDP and an increase in typical apoptotic morphological changes were observed in OMT plus CDDP group (Fig 3a-b). Next, we quantitatively examined the effects of OMT and CDDP using flow cytometry assay. Result showed that either OMT or CDDP alone induced apoptosis in BGC823 cells, and the co-treatment with OMT and CDDP caused a greater increase in the rate of apoptosis (Fig 3c-d). To analyze OMT- and CDDP-induced apoptosis, we assessed AKT/ERK activation by western blotting analysis. The result demonstrated that the co-treatment with OMT and CDDP significantly inhibited the phosphorylation of AKT and ERK in BGC823 cells (Fig 6).
OMT synergistically potentiates CDDP-induced cell proliferation inhibition. (a) MTT assay was used to measure the cell viability of BGC823 and SGC7901 cells after the treatment of CDDP. (b,c) The BGC823 and SGC7901 cell viability after OMT and/or CDDP treatment. (d,e) Colony formation of BGC823 cells after OMT and/or CDDP treatment. *P < 0.01 versus the control group; **P < 0.01 versus OMT or CDDP alone group.
OMT enhances CDDP-induced apoptosis in BGC823 cells. (a) Hoechst 33342 staining was used to observe the nuclear condensation and cell morphology changes in BGC823 cells after OMT plus CDDP treatment (original magnification×200). (b) The percentage of apoptosis cells was calculated as apoptosis index (AI) (%) and shown in histograms. (c,d) After co-treatment with OMT and CDDP, cell apoptosis was observed by flow cytometry and the apoptosis rate of BGC823 cells was shown. *P<0.01 versus the control group; **P < 0.01 versus OMT or CDDP alone group.
Effects of OMT and/or CDDP on BGC823 cell cycle distribution and invasion. (a) Cell cycle analysis of BGC823 cells was detected by FACS. Quantification of the distribution of cell cycle was shown in (b). (c) After incubation with OMT and/or CDDP, the invasive property of BGC823 cells was tested in transwell plates (original magnification 200×). (d) The number of invasive cells. *P < 0.01 versus the control group; **P < 0.01 versus OMT or CDDP alone group
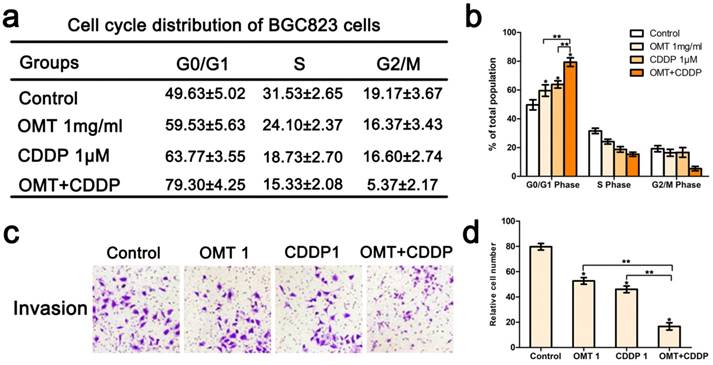
Co‑treatment of OMT and CDDP synergistically induced cycle arrest and inhibited invasion of GC cells
We next applied FCM to analyze the cell cycle phases of the treated BGC823 cells. The results showed that there was an accumulation of cell population in G0/G1-phase after OMT or CDDP treatment. OMT plus CDDP treatment group revealed a significantly greater proportion of cells in G0/G1 phase. Figure 4c-d showed that the invasive cell numbers were significantly decreased after OMT or CDDP treatment. Meanwhile, the combination treatment showed the least invasive cell number. Western blotting analysis was performed to investigate the expression of cyclin D1, p21 and p27. We found that after the single drug treatment, cyclin D1 was significantly down-regulated, whereas p21 and p27 were significantly up-regulated, and the drug combination treatment group showed the most significant difference (Fig 6).
OMT and/or CDDP triggered ROS generation in GC cells
We next investigated intracellular ROS levels in BGC823 cells treated with OMT and/or CDDP using the DCFH-DA assay. As shown in Figure 5a-b, OMT or CDDP treatment increased ROS generation in BGC823 cells compared to the control group and the effect was much more pronounced in the co-treatment group. Next, we detected the effects of ROS scavenger NAC, in combination with OMT, CDDP, or OMT + CDDP for 24h. NAC reduced the anti-proliferative effects of OMT, CDDP, and OMT plus CDDP, suggesting that ROS may play a pivotal role in the synergism between OMT and CDDP (Fig 5c).
OMT treatment improved the antitumor effect of CDDP in vivo
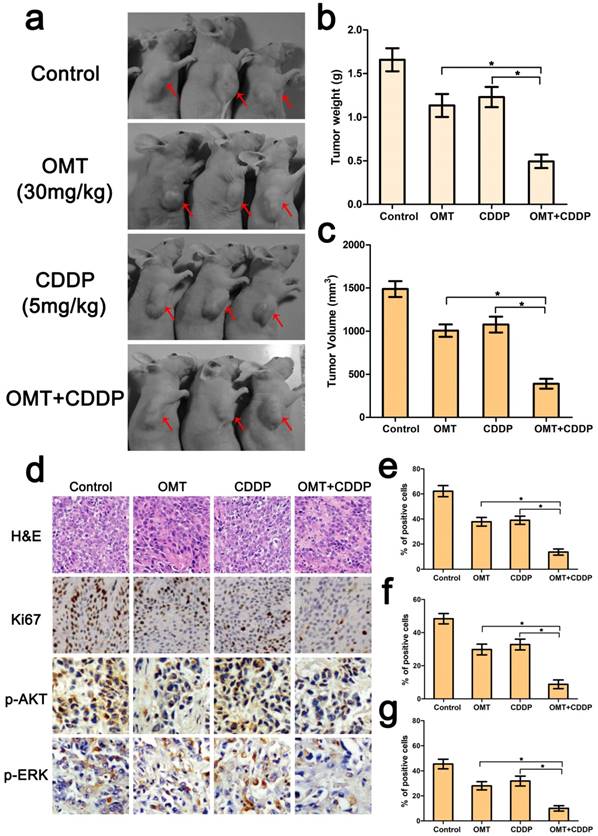
Because of the superior anti-tumor effects of the OMT+CDDP in vitro, we investigated whether the co-treatment group inhibited tumor growth in vivo. Results showed that co-treatment with both agents exerted marked anticancer activity in BGC823 xengraft tumors. We also calculated the mean tumor weights in different groups, and found that the tumor weight inhibition rates were 31.52%, 25.81% and 70.27% for OMT, CDDP, and OMT plus CDDP group. The trends of tumor volume inhibition were similar to tumor weight inhibition.
Further, HE staining demonstrated that the structure of tumor tissue was more serious damaged and the major of the cells exhibited condensed nuclei and a reduction in nuclear staining after OMT+CDDP treatment (Fig 7d). In addition, we examined the expressions of Ki67, p-AKT, p-ERK by immunohistochemistry. As shown in Figure 7d-g, OMT plus CDDP significantly decreased the mean areas that stained positively for Ki67, p-AKT and p-ERK compared with OMT or CDDP alone group (P < 0.01).
Discussion
Many GC patients have died of drug-resistant recurrence although continuous efforts have been made in early diagnosis and therapy [4, 21]. Nowadays, cancer chemotherapy using plant-derived active components in combination with anti-cancer drugs have attracted more and more attention owing to its effectiveness and improved safety [8, 22]. In present study, we found that OMT significantly suppresses GC cell growth, induces cell apoptosis, and displays a magnificent chemo-sensitization effect to potentiate the CDDP-induced cell proliferation inhibition. Therefore, it would be a promising approach to use the combination of OMT and CDDP for treatment of GC.
Cell apoptosis is a type of programmed cell death and the induction of apoptosis plays a critical role in tumor therapy [23, 24]. Apoptosis is typically induced through the extrinsic and intrinsic pathways [25]. The reason of tumor drug-resistance has mainly been attributed to anti-apoptotic pathways and the sensitivity of cancer cells to chemotherapeutic drug-induced apoptosis [26]. It has been reported that the regulation of the cell cycle is closely related to cell apoptosis and proliferation of cancers [27, 28]. It is obviously that an agent who could induce cancer cells apoptosis and cycle arrest would be a hopeful candidate to inhibit cancer progression. In this study, we found that the combination of OMT and CDDP showed stronger inhibitory effect than single agent alone in GC cells. Combination index data analysis demonstrated that OMT combined with CDDP has synergistic effect against GC cells. Furthermore, there was an accumulation of cell population in G0/G1 phase after OMT treatment, and OMT significantly enhanced the CDDP caused cell cycle arrest in G0/G1 phase by increasing p27, p21 and decreasing cyclin D1.
OMT and CDDP act synergistically to up-regulating ROS levels in BGC823 cells. (a) Intracellular ROS detection after treated with OMT, CDDP, or OMT plus CDDP. (b) Quantitative analysis of intracellular ROS levels by flow cytometry. (c) Analysis of cell survival after dealt with OMT and/or CDDP in the presence or absence of NAC. *P < 0.01, versus the control group; **P < 0.01, versus OMT or CDDP alone group; #P < 0.05; ##P < 0.01 versus the group that treatment without NAC.
OMT and CDDP act synergistically to inhibit the AKT/ERK pathway. (a) Western blotting assay was used to analysis the expression level of cyclin D1, p21, p27, AKT, p-AKT, ERK and p-ERK. (b) The densitometry analysis of every factor was performed, normalized with the corresponding GAPDH content. *P < 0.01 versus OMT or CDDP alone group.
Combination of OMT and CDDP arrests tumor growth. (a) Representative images of the tumors in each group were shown. (b,c) The final tumor weight and volume in every groups after different treatments. (d) The expression levels of Ki67, p-AKT, and p-ERK in xenograft tumors were determined by immunohistochemical analysis (magnifcation, 200× for Ki67 and 400× for p-AKT, p-ERK). (e-g) Expression of Ki67, p-AKT, and p-ERK was quantified in percentages of positive cells within five medium-power fields were shown in histograms. *P < 0.01 versus OMT or CDDP alone group.
ROS and tumor biology are intertwined [29]. ROS plays an important role in cellular function and survival signaling, and the accumulation of intracellular ROS could finally activate apoptosis signaling pathways [30]. Recent studies have demonstrated the importance of ROS in therapeutic approaches to effectively kill various tumor cells [31]. Also ROS can indirectly mediate apoptosis through the MAPK, JNK and AKT/ERK pathways [32]. The AKT/ERK signaling pathway has crucial roles in maintenance of cell growth, induction of cell apoptosis and cycle arrest [33, 34]. It can suppress apoptosis by directly phosphorylating apoptotic signaling proteins and alterations of this pathway have been observed in many types of tumors [35, 36]. CDK4-Cyclin D complex is a major integrator of cell proliferation and it could regulate cell cycle [37]. Cyclin D is regulated by the upstream pathways including AKT/ERK pathway. Blockage of the AKT/ERK pathway results in programmed cell death and growth inhibition of cancer cells. Here, we found that OMT and/or CDDP increased the intracellular ROS level in BGC823 cells, and co-treatment group induced higher ROS accumulation than single agent alone. Importantly, NAC pre-treatment almost completely reversed cell apoptosis. The levels of activated AKT and ERK were obviously decreased after OMT and/or CDDP treatment. These results indicated that ROS generation may play a key role in cell growth inhibition and apoptosis of GC cells.
In addition, we further found that either OMT or CDDP could inhibit the tumor growth during the treatment period, and the combination group induced a very robust anticancer activity. H&E staining analysis demonstrated that CDDP induced BGC823 cells apoptosis and this effect was enhanced by OMT. Moreover, immunohistochemical analysis proved the decrease of p-AKT, p-ERK, and Ki67 following treatment with OMT and/or CDDP, which was consistent with our findings in vitro.
In summary, we have demonstrated that OMT synergistically potentiates the effect of CDDP against GC cells by inhibiting cell viability and invasion, and inducing cell apoptosis and cycle arrest. More importantly, these effects were mediated by inactivation of the AKT/ERK pathway and ROS generation. Overall, these results indicate that co-treatment with OMT and CDDP may serve as a promising strategy for GC treatment.
Abbreviations
CDDP: Cisplatin; DMSO: Dimethyl sulfoxide; FBS: Fatal bovine serum; GC: Gastric Cancer; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; MAPK: Mitogen-activated protein kinase; MTT: 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide; NAC: Nacetylcysteine; OMT: Oxymatrine; PBS: Phosphate-buffered saline; PVDF: Polyvinylidene difluoridex; SDS-PAGE: Sodium dodecylsulphate polyacrylamide gel electrophoresis; TBST: Tris buffer solution tween.
Acknowledgements
The present study was supported by the Program for Medical and health application basic research of Suzhou City (No.SYSD2016042), the Program for Wujiang No.1 People's Hospital (No.201603).
Authors' contributions
LY, BTT and QGQ carried out the main work and drafted the manuscript. DW and LW carried out the western blotting and immunohistochemistry. QL and QLQ carried out the in vivo study. LY, SGH performed the statistical analysis. GQG, SGH and LY designed the study. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Torre LA, Bray F, Siegel RL. et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108
2. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202-209
3. Kim YI, Choi IJ, Kook MC. et al. The association between Helicobacter pylori status and incidence of metachronous gastric cancer after endoscopic resection of early gastric cancer. Helicobacter. 2014;19:194-201
4. Marin JJ, Al-Abdulla R, Lozano E. et al. Macias mechanisms of resistance to chemotherapy in gastric cancer. Anticancer Agents Med Chem. 2016;16:318-334
5. Yawata K, Osada S, Tanahashi T. et al. The significant role of cyclin D1 in the synergistic growth-inhibitory effect of combined therapy of vandetanib with 5-fluorouracil for gastric cancer. Anticancer Res. 2016;36:5215-5226
6. Pasini F, Fraccon AP, DE Manzoni G. The role of chemotherapy in metastatic gastric cancer. Anticancer Res. 2011;31:3543-3554
7. Koizumi W, Narahara H, Hara T. et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215-221
8. Zhou D, Liu W, Liang S. et al. Apoptin-derived peptide reverses cisplatin resistance in gastric cancer through the PI3K-AKT signaling pathway. Cancer Med. 2018;7:1369-1383
9. Guzman JR, Koo JS, Goldsmith JR. et al. Oxymatrine prevents nf-kappab nuclear translocation and ameliorates acute intestinal inflammation. Sci Rep. 2013;3:1629
10. Chen H, Zhang J, Luo J. et al. Antiangiogenic effects of oxymatrine on pancreatic cancer by inhibition of the nf-kappab-mediated vegf signaling pathway. Oncol Rep. 2013;30:89-595
11. Song MQ, Zhu JS, Chen JL. et al. Synergistic effect of oxymatrine and angiogenesis inhibitor nm-3 on modulating apoptosis in human gastric cancer cells. World J Gastroenterol. 2007;13:1788-1793
12. Zhang Y, Piao B, Zhang Y. et al. Oxymatrine diminishes the side population and inhibits the expression of beta-catenin in mcf-7 breast cancer cells. Med Oncol. 2011;28(Suppl 1):S99-107
13. Zhang Y, Sun S, Chen J. et al. Oxymatrine induces mitochondria dependent apoptosis in human osteosarcoma MNNG/HOS cells through inhibition of PI3kAKT pathway. Tumour Biol. 2014;3:1619-1625
14. Liu Y, Bi T, Dai W. et al. Oxymatrine synergistically enhances the inhibitory effect of 5-fluorouracil on hepatocellular carcinoma in vitro and in vivo. Tumour Biol. 2016;37:7589-7597
15. Liu Y, Bi T, Wang Z. et al. Oxymatrine synergistically enhances antitumor activity of oxaliplatin in colon carcinoma through PI3K/AKT/mTOR pathway. Apoptosis. 2016;21:1398-1407
16. Kawahara T, Inoue S, Ide H. et al. ZKSCAN3 promotes bladder cancer cell proliferation, migration, and invasion. Oncotarget. 2016;7:53599-53610
17. Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440-446
18. Liu Y, Bi T, Wang G. et al. Lupeol inhibits proliferation and induces apoptosis of human pancreatic cancer PCNA-1 cells through AKT/ERK pathways. Naunyn Schmiedebergs Arch Pharmacol. 2015;388:295-304
19. Liu Y, Bi T, Shen G. et al. Lupeol induces apoptosis and inhibits invasionin gallbladder carcinoma GBC-SD cells by suppression of EGFR/MMP-9 signaling pathway. Cytotechnology. 2016;68:123-133
20. Liu Y, Bi T, Dai W. et al. Lupeol enhances inhibitory effect of 5-fluorouracil on human gastric carcinoma cells. Naunyn-Schmiedebergs Arch Pharmacol. 2016;389:477-484
21. Zhang X, Yao J, Guo K. et al. The functional mechanism of miR-125b in gastric cancer and its effect on the chemosensitivity of cisplatin. Oncotarget. 2017;9:2105-2119
22. Lian G, Li L, Shi Y. et al. BI2536, a potent and selective inhibitor of polo-like kinase 1, in combination with cisplatin exerts synergistic effects on gastric cancer cells. Int J Oncol. 2018;52:804-814
23. Savitskaya MA, Onishchenko GE. Mechanisms of apoptosis. Biochemistry (Mosc). 2015;80:1393-1405
24. Oral O, Akkoc Y, Bayraktar O. et al. Physiological and pathological significance of the molecular cross-talk between autophagy and apoptosis. Histol Histopathol. 2016;31:479-498
25. Cao L, Quan XB, Zeng WJ. et al. Mechanism of hepatocyte apoptosis. J Cell Death. 2016;9:19-29
26. Kelly PN, Strasser A. The role of Bcl-2 and its pro-survival relatives in tumourigenesis and cancer therapy. Cell Death Differ. 2011;18:1414-1424
27. Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456-1462
28. Wang L, Wu J, Lu J. et al. Regulation of the cell cycle and PI3K/Akt/mTor signaling pathway by tanshinone I in human breast cancer cell lines. Mol Med Rep. 2015;11:931-939
29. Tong L, Chuang CC, Wu S. et al. Reactive oxygen species in redox cancer therapy. Cancer Lett. 2015;367:18-25
30. Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signaling pathways by reactive oxygen species. Biochim Biophys Acta. 2016;1863:2977-2992
31. Ozben T. Oxidative stress and apoptosis: impact on cancer therapy. J Pharm Sci. 2007;96:2181-2196
32. Wu CC, Bratton SB. Regulation of the intrinsic apoptosis pathway by reactive oxygen species. Antioxid Redox Signal. 2013;19:546-558
33. Liang J, Slingerland JM. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle. 2003;2:339-345
34. Kim DJ, Reddy K, Kim MO. et al. (3-Chloroacetyl)-indole, a novel allosteric AKT inhibitor, suppresses colon cancer growth in vitro and in vivo. Cancer Prev Res (Phila). 2011;4:1842-1851
35. Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424-430
36. Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene. 2004;23:2838-2849
37. Alao JP. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer. 2007;6:24
Author contact
![]() Corresponding authors: Dr Quangen Gao (dzghcccs.edu), Genhai Shen (wjyysghcom), Department of General Surgery, Wujiang No.1 People's Hospital affiliated to Nantong University, Suzhou, Jiangsu 215200, PR China Phone (Fax): +8652163426145
Corresponding authors: Dr Quangen Gao (dzghcccs.edu), Genhai Shen (wjyysghcom), Department of General Surgery, Wujiang No.1 People's Hospital affiliated to Nantong University, Suzhou, Jiangsu 215200, PR China Phone (Fax): +8652163426145

 Global reach, higher impact
Global reach, higher impact