3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2021; 12(17):5268-5274. doi:10.7150/jca.60014 This issue Cite
Research Paper
Continuing Cetuximab vs Bevacizumab plus chemotherapy after first progression in wild-type KRAS, NRAS and BRAF V600E metastatic colorectal cancer: a randomized phase II trial
1. Department of Oncology, Affiliated cancer hospital of Zhengzhou University, Henan Cancer Hospital, Zhengzhou, China 450008.
2. Department of Oncology, The first affiliated hospital of Zhengzhou University, Zhengzhou, China 450052.
3. Department of Oncology, Henan Province Hospital, Zhengzhou, China 450003.
Abstract
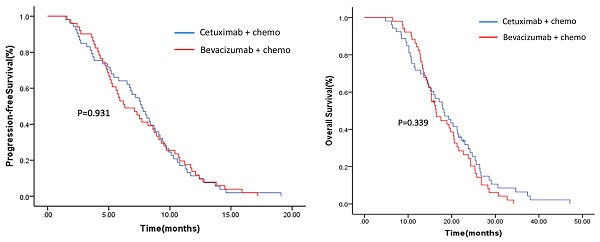
To evaluate the clinical efficacy of continuing cetuximab vs bevacizumab plus chemotherapy crossover after first progression to cetuximab regimen in wild-type KRAS, NRAS and BRAF V600E mCRC, we conducted this prospective, open-label and randomized phase 2 trial in three cancer centers from Oct 1, 2016 to July 1, 2020. Eligibility criteria included documented progressive disease during or after first-line treatment with cetuximab regimen; second biopsy confirmed as KRAS, NRAS and BRAF V600E wild-type mCRC. Patients were randomized to arm A (cetuximab+chemo) or arm B (bevacizumab+chemo) with second-line chemotherapy crossover. The primary end point was progression free survival (PFS). Secondary end points included objective response rate (ORR), overall survival (OS) and toxicity. Tissue VEGFA, ERBB2 and MET mRNA were examined by real time RT-PCR.
A total of 104 patients (53 in arm A and 51 in arm B) were enrolled. Median PFS was 7.7 months (95% CI: 6.5-8.9) for arm A and 6.3 months (95% CI: 4.5-8.1) for arm B (p=0.931). Median OS was 18.2 months (95% CI: 14.5-21.9) for arm A and 16.4 months (95% CI: 14.2-18.6) for arm B (p=0.339). The ORR was 28.3% and 19.6% in arm A and arm B (p=0.31), respectively. MET mRNA was highly expressed in the cetuximab-progressed tumors, but treatment responsiveness to cetuximab or bevacizumab in each arm was not correlated with the MET expression level. The results showed no significant difference in PFS, OS and ORR between the two arms, but a trend in favor of the cetuximab continuation plus chemotherapy crossover was examined in all end points. High expression of MET in cetuximab-progressed tumors may indicate an existence of MET-dependent tumor cell population.
Keywords: metastatic colorectal cancer, Cetuximab first progression, RAS wild type, MET expression

 Global reach, higher impact
Global reach, higher impact