3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(11):3209-3220. doi:10.7150/jca.76695 This issue Cite
Review
KRAS as a Key Oncogene in the Clinical Precision Diagnosis and Treatment of Pancreatic Cancer
1. Department of Hepatobiliary Surgery, Hunan Provincial People's Hospital, The First Affiliated Hospital of Hunan Normal University, Changsha, 410005 Hunan Province, China.
2. Xiangyue Hospital Affiliated to Hunan Institute of Parasitic Diseases, National Clinical Center for Schistosomiasis Treatment, Yueyang 414000, Hunan Province, China.
3. Translational Medicine Laboratory of Pancreas Disease of Hunan Normal University, Changsha 410005, China.
Abstract
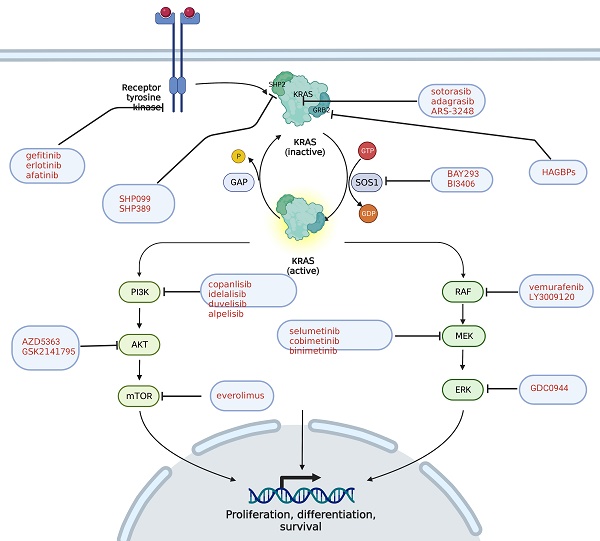
Pancreatic ductal adenocarcinoma (PDAC) is one of the most malignant tumors, with a 5-year survival rate of less than 10%. At present, the comprehensive treatment based on surgery, radiotherapy and chemotherapy has encountered a bottleneck, and targeted immunotherapy turns to be the direction of future development. About 90% of PDAC patients have KRAS mutations, and KRAS has been widely used in the diagnosis, treatment, and prognosis of PDAC in recent years. With the development of liquid biopsy and gene testing, KRAS is expected to become a new biomarker to assist the stratification and prognosis of PDAC patients. An increasing number of small molecule inhibitors acting on the KRAS pathway are being developed and put into the clinic, providing more options for PDAC patients.
Keywords: Pancreatic neoplasms, KRAS gene, Targeted therapy, Diagnosis, Review

 Global reach, higher impact
Global reach, higher impact