Impact Factor
ISSN: 1837-9664
J Cancer 2026; 17(2):382-394. doi:10.7150/jca.126397 This issue Cite
Research Paper
Comparative Blood-Based Transcriptomic Profiles of Prostate Cancer Patients from South Africa and the USA: A Cross-Sectional Pilot Study
1. Wren Laboratories LLC, Branford CT 06405, USA.
2. Faculty of Health Sciences, University of Pretoria, Pretoria, South Africa
3. Department of Integrative Biological Sciences, Institute of Infectious Diseases and Molecular Medicince, University of Cape Town.
Received 2025-10-8; Accepted 2026-1-8; Published 2026-1-14
Abstract
Prostate cancer (PCa) is a major health problem worldwide with variable incidence, progression and outcomes depending on genetic, environmental and socio-economic factors. This study compares gene expression profiles in PCa patients from South Africa (RSA) and the United States (USA) using RNA sequencing in whole blood and pathway analyses. Whole blood samples were collected in Wren RNA stabilization tubes from RSA-PCa (n = 6), RSA-controls (n = 6), USA-PCa (n = 7) and USA-Controls (n = 11). RNA sequencing revealed 1,627 differentially expressed genes (DEGs) in RSA-PCa vs. RSA-controls, and 2,193 DEGs in USA-PCa vs. USA-Controls. Pathway analyses identified geographical region-specific variations; RSA-PCa had upregulated myeloid suppressor cell pathways and immunosuppressive markers while USA-PCa samples exhibited upregulated cytokine signaling and inflammatory pathways. Comparative analysis of healthy controls revealed 2,280 DEGs, which indicated significant differences in molecular profile of the geographic locations. qRT-PCR undertaken on 27 biomarkers related to PCa in whole blood (PROSTest) identified that 26 (96%) of the marker genes were commonly expressed. RNAseq and normalized PCR gene expression of these markers were well-correlated (r = 0.44, p = 0.0012, n = 30 pairs). The results of this study indicate that there are geographic differences in blood-based gene expression in both controls and individuals with PCa. Genes associated with a clinically validated molecular assay (PROSTest) were identified in both populations, but significant differences in gene expression relevant to tumor pathobiology were identified. These immune-associated signaling pathways suggest differences between these two cohorts in blood-based molecular architecture related to PCa. They also suggest the need to consider population-specific biomarkers to better understand this disease. Ultimately, optimizing blood-based molecular diagnostic and therapeutic approaches will require population-level studies.
Introduction
Prostate cancer (PCa) is a significant public health concern and one of the most frequently diagnosed malignancies in men worldwide. It is the second most common cancer in men after lung cancer, and ~1.5 million new cases were diagnosed in 2022 [1]. The United States has the highest incidence; 313, 780 new cases were diagnosed in 2025, and the lifetime risk of being diagnosed with PCa is about 1 in 8 [2].
PCa risk varies significantly across geographic, socioeconomic, and racial contexts. Predominant risk factors include age (from ~5% in < 30-year-old males to ~60% by age of 80), lifestyle (BMI and high alcohol intake, socioeconomic status), genetic burden (family history, inherited mutations in DNA damage and mismatch repair genes), and ethnicity [3-5]. Men of African descent have a significantly elevated risk (2-3-fold) of having PCa compared to Caucasian, Hispanic/Latino and Asian men [6]. In South Africa, PCa is the most commonly diagnosed cancer in African men, with a 60% increase in new cases reported between 2002 and 2018 [7]. This is attributed to increasing life expectancy, improved diagnostic capabilities, lifestyle changes, and environmental exposures [8]. Disparities in healthcare access, such as unequal availability of diagnostic tools and treatment facilities, further exacerbate mortality rates in low-resource regions. These differences underscore the need for equitable diagnostic strategies and region-specific research to address the unique genetic and environmental factors influencing PCa [7].
Advances in next-generation sequencing (NGS) have transformed PCa research, offering insights into the molecular mechanisms of the disease [9]. NGS facilitates comprehensive profiling of genetic and transcriptomic alterations, enabling the discovery of novel biomarkers for early detection, disease monitoring, and personalized treatment. Molecular testing, typically used at a tissue level, includes the development of several mRNA-based tools e.g., Oncotype Dx, that are now used in PCa management. Peripheral blood, however, provides an alternative testing compartment for RNA-based assays [10]. CTCs and cfRNA are detectable in PCa blood [11], while platelets are a well-known source of tumor-derived mRNA [12]. Whole blood-derived gene expression assays have been described [13, 14]; different immune-related 6- and 9-gene or PCa tumor-associated 5-gene assays are available for research purposes [13-15]. PROSTest is a clinically validated liquid biopsy that is a minimally invasive, mRNA-based diagnostic (27 target genes) with > 90% sensitivity that leverages molecular profiling of gene expression in whole blood samples [16, 17]. While providing a source of “tumor signal”, peripheral blood also offers an opportunity to interrogate molecular information from circulating immune cells. This may provide insights into host and environmental responses.
In this study, we evaluated whether blood-based gene expression patterns in PCa between Caucasian US and Black South African populations was different. We posited than any differences could reflect variations in underlying genetic and environmental factors which are known to exist [8, 18, 19]. Using RNA sequencing and liquid biopsy technologies, we compared transcriptomic signatures in two age- and tumor biology-matched sample sets as well as the clinically validated PROSTest PCR-based signature across these populations. The objective was to use these tools to provide insights into geographic/ethnic-specific disease pathobiology of PCa. We undertook this global approach but also focused on genes and pathways e.g., AR-signaling, known to differ between these ethnicities [20-22].
Materials and Methods
Clinical Samples: Based on established protocols for RNAseq-based DEGs [23], we focused on a minimum of 6 biological samples from each of the 4 groups (PCa-USA, PCa-RSA, Con-USA, Con-RSA), that we wanted to evaluate. Whole blood samples were collected between 2020-24 [United States (USA-PCa: 7, USA-Control: 11), Republic of South Africa (RSA-PCa: 6, RSA-Control: 6)] in accordance with respective guidelines and regulations of both countries (United States: WIRB20191743 approved by the Western Institutional Review/WCG Board; South Africa: 389/2020 approved by the Research Ethnics Committee of the University of Pretoria, Reference number: GP202009 032 on the South African Health Research Database) and with the Helsinki declaration of 1975, as revised in 2013. All samples were collected from adult participants [USA median age: 61.5 yrs (USA-PCa: 66 yrs; USA-Controls: 57 yrs); RSA median age: 57.5 yrs (RSA-PCa: 63 yrs; RSA-Controls: 66 yrs)] and informed consent was obtained from all subjects in accordance with ethnical guidelines. All patients age, ethnicity, Gleason scores, disease status with various stages in Table 1.
RNA isolation: Total RNA was extracted from whole blood (at Wren Laboratories LLC, USA) using TRIzol (Thermofisher) and cleaning further by RNeasy Mini isolation kit (Qiagen) [24, 25]. Briefly, 150 μl of whole blood was added to 750 μl of TRIzol LS, incubated for 5 minutes, vortexed with 200 μl of chloroform, and centrifuged at 13,000 rpm for 12 minutes. The aqueous phase was transferred to a new 2 ml sample tube and RNA isolation and purification was carried out using the QIACube MDx instrument (Qiagen) with RNeasy Mini Kit according to the manufacturer's instructions. RNA was eluted in 80 μl of nuclease-free water.
RNA-seq quality control: RNA quality was assessed using the A260/A280 and A260/A230 ratios measured by a NanoDrop spectrophotometer. RNA integrity was evaluated with an Agilent Bioanalyzer 2100, and samples with RIN values of 6.5 or greater were included in library preparation.
RNA seq library prep: mRNA was enriched from approximately 50 ng of total RNA using oligo-dT beads and fragmented by incubation at 94ºC in the presence of Mg2+ (Kapa mRNA Hyper Prep). First-strand cDNA synthesis was performed using random primers, followed by second-strand synthesis and A-tailing with dUTP to generate strand-specific sequencing libraries. Adapter ligation with 3' dTMP overhangs were ligated to library insert fragments. Library amplification amplifies fragments carrying the appropriate adapter sequences at both ends. Strands marked with dUTP were not amplified. Libraries were sequenced on an Illumina NovaSeq 6000 platform to generate 25 million reads per sample for further analysis (Yale University Core, USA).
Flow cell preparation and sequencing: Sample concentrations were normalized to 1.2 nM and loaded onto an Illumina NovaSeq flow cell at a density targeting 2 million passing filter clusters per sample. Sequencing was performed using 100 bp paired-end reads in accordance with Illumina protocols. A 10 bp unique dual index was used for sample identification. Positive control libraries (PhiX, 0.3%) were spiked in to monitor sequencing quality.
Data extraction: Signal intensities are converted to individual base calls during a run using the system's Real-Time Analysis software. Base calls were transferred from the machine's dedicated personal computer to the Yale High Performance Computing cluster via a 1 Gigabit network mount for downstream analysis. Primary analysis, including demultiplexing and genome alignment, was conducted using CASAVA 1.8.2 software.
mRNA-seq data analysis: FASTQ mRNA-Seq files were processed with Partek Flow® software, version 11.0.23.0918 (Partek, Inc., St. Louis, MO) on a Linux-based high performance computing system at Partek (40 cores, 503 GB RAM, 4TB disk space). The adapter was removed with CUTADAPT v4.2 and remapped into the human hg38 genome using the BWA aligner (v0.7.17) with default settings (minimum bases 24 read length) for read mapping [24, 26, 27]. Gene annotation was performed using Ensembl v109, and transcript abundance was normalized with DESeq2 for statistical analysis with FDR correction. DEGs were identified using a threshold of p < 0.05 and fold-change > 2 [24]. Partek Flow is commercially available graphical interface web-based application, which allows the choice of variables and parameters for typical data analysis.
Clinical information of recruited patients
| Patient No | Age | Diagnosis | Ethnicity | Status | Stage at Diagnosis | Gleason Score | Grade at Diagnosis | Staging (T) | Staging (N) | Staging (M) |
|---|---|---|---|---|---|---|---|---|---|---|
| USA-P1 | 76 | PCa | C | ND | IC | 7A | GG2 | cT1c | NX | N/A |
| USA-P2 | 68 | PCa | C | ND | IIB | 7B | GG3 | cT2b | cN0 | M0 |
| USA-P3 | 60 | PCa | C | ND | IC | 7B | GG3 | cT1c | NX | N/A |
| USA-P4 | 58 | PCa | C | AS | I | 6 | GG1 | cT1 | cN0 | cM0 |
| USA-P5 | 64 | PCa | C | AS | I | 6 | GG1 | pT1c3 | N0 | M0 |
| USA-P6 | 57 | PCa | C | ND | N/A | 7A | GG2 | pT3a | pN0 | N/A |
| USA-P7 | 44 | PCa | C | ND | N/A | 7A | GG2 | pT2 | N0 | N/A |
| RSA-P1 | 60 | PCa | B | ND | T2A | 6 | GG1 | pT2 | N0 | N/A |
| RSA-P2 | 68 | PCa | B | ND | T1C | 6 | GG1 | pT2 | N0 | N/A |
| RSA-P3 | 61 | PCa | B | AS | T2C | 7A | GG2 | pT3a | pN0 | N/A |
| RSA-P4 | 58 | PCa | B | AS | T1C | 6 | GG1 | cT1 | cN1 | cM0 |
| RSA-P5 | 66 | PCa | B | AS | T4 | 7A | GG2 | pT3a | pN0 | N/A |
| RSA-P6 | 66 | PCa | B | AS | T4 | 8 | G4 | pT3a | pN0 | N/A |
| USA-Healthy1 | 52 | Control | C | |||||||
| USA-Healthy2 | 57 | Control | C | |||||||
| USA-Healthy3 | 56 | Control | C | |||||||
| USA-Healthy4 | 54 | Control | C | |||||||
| USA-Healthy5 | 65 | Control | C | |||||||
| USA-Healthy6 | 58 | Control | C | |||||||
| USA-Healthy7 | 64 | Control | C | |||||||
| USA-Healthy8 | 54 | Control | C | |||||||
| USA-Healthy9 | 78 | Control | C | |||||||
| USA-Healthy10 | 45 | Control | C | |||||||
| USA-Healthy11 | 63 | Control | C | |||||||
| RSA-Healthy1 | 83 | Control | B | |||||||
| RSA-Healthy2 | 60 | Control | B | |||||||
| RSA-Healthy3 | 64 | Control | B | |||||||
| RSA-Healthy4 | 64 | Control | B | |||||||
| RSA-Healthy5 | 57 | Control | B | |||||||
| RSA-Healthy6 | 83 | Control | B |
GG = Gleason Grade, ND = no data, C = Caucasian, B = Black, AS = Active Surveillance; ND = Newly Diagnosed, N/A = not available.
Pathway analysis: Pathway analysis was carried out using publicly available tools, including Metascape, Gene Ontology (GO), SRplots [28, 29] and g:Profiler. Functional analysis encompassed GO Molecular Functions, Biological Processes, Cellular Components, and pathway enrichment analysis were carried out on clusterProfiler and pathview [27, 30, 31]. The results provided insights into molecular dysregulations associated with PCa across USA and RSA cohorts.
Real-Time qPCR: The above clinical samples RNA was converted into cDNA using High-Capacity cDNA Reverse Transcriptase Kit (ThermoFisher). Real-time PCR was carried out on pre-spotted TaqMan PCR primer plates [27 PROSTest genes (list of genes in Table 3)] using 200 ng of cDNA/well [16, 17]. Positive controls were used on every spotted plate. Target transcript levels were normalized to ALG9, TOX4 and TPT1 and quantified using ΔΔCT.
Immune cell analysis: Immune infiltration estimations for gene expression data was carried out using web-based tools including Immunedevconv with TIMER 2.0, CIBERSORT, quanTIseq, xCell, MCP-counter and EPIC algorithms [32-35].
Statistics and data visualization: All experiments were minimum of triplicates to validate reproducibility and p-value < 0.05 considered statistically significant (for RNA-seq, FDR corrected). Circos plots were used as they are a powerful visualization tool used to display complex relationships within large-scale RNA-seq data in circular layout with chromosome number and transcript position to identify gene expression hotspots [36].
Results
Clinical Data: A total of 13 PCa (7 USA, 6 RSA) and 17 controls (11 USA, 6 RSA) were evaluated. There was no significant difference in median ages (USA-PCa: 60 yrs, RSA-PCa: 63.5 yrs, USA-Con: 57 yrs, RSA-Con: 64 yrs; Kruskal-Wallis test: p = 0.18). PCa patients were comparable in the two cohorts. The distribution of low grade (GG1-2) Gleason grade (USA-PCa: 5/7; RSA-PCa: 5/6) and stage I-II disease (USA-PCa: 5/7; RSA-PCa4/6) were similar in both cohorts. All patients were newly diagnosed (7/13 [54%]) or in an active surveillance program (6/13 [46%]). None were currently being treated.
Cancer-related peripheral blood differences
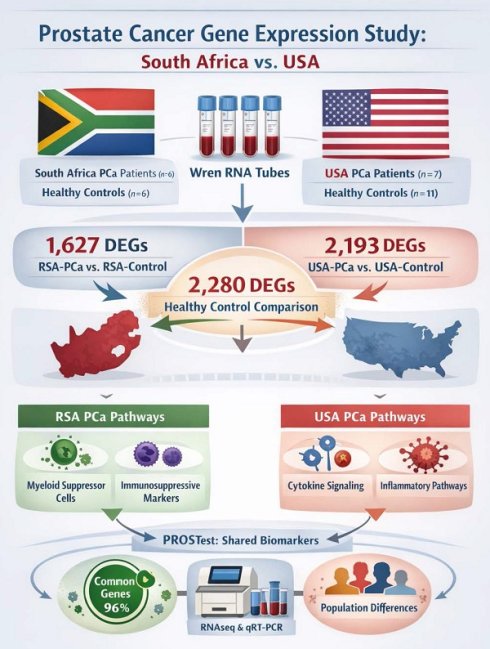
We initially compared RSA-PCa and USA-PCa sample sets to identify differences in blood-derived gene expression between subjects with PCa from these two different geographic regions. A total of 7,149 genes were identified to be differentially expressed with a FC > 2 (p < 0.05) (Figure 1A). The five most significant genes that were upregulated in RSA-PCa with the highest fold changes included CERS6-AS1 (FC:238.1), MTCO1P40 (FC:107.5), ATP6V1G2-DDX39B (FC:53.4), MTND4P12 (FC:34.0), RMRP (FC:30.1), while the most down regulated genes were GVINP1 (FC:-702.0), ENSG00000273217 (FC:-56.3; Rap Guanine Nucleotide Exchange Factor 6, associated with obesity and cholesterol metabolism), ENSG00000263244 (FC:-29.2; a novel transcript, 3' overlapping Chromosome 16 ORF72, associated with obesity and cholesterol metabolism), LY75-CD302 (FC:-26.5) and CXCL8 (FC:-10.6).
Figure 1B is a Circos plot that identified DEG hotspots in Chromosome 1, 2, and 17. None of these are associated with known genetic (GWAS) differences between Caucasians and Blacks [37] or were associated with the 27 PROSTest marker genes.
Pathway analyses (Figure 1C) identified the differences in Molecular adapter activity (signaling pathways), Molecular Carrier Activity (biomolecular transport), and Ubiquitin-Ubiquitin Ligase Activity. Biological processes including Cell Division, Mitochondrial Electron Transport: Growth Hormone Receptor Signaling and Fibroblast Proliferation were also noted to be different. These processes can all influence the tumor microenvironment, potentially impacting PCa development and progression.
PCa-associated genes known to be differentially expressed (between Caucasians and Blacks) at a tissue level [20] was then examined in the blood samples. We focused on the 10 most differentially expressed (up/down) identified by Kim et al. [20]. Seven of the 20 were differentially expressed in blood (Figure 1D, Supplementary Table 1). Elevated expression (consistent with tumor tissue) was noted for IGKV2-29 (FC: 4.39), a B cell/plasma cell-related gene associated with the tumor microenvironment. STEAP4 (FC: -1.44) and SH3KBP1 (FC: -1.26) were decreased. Both are associated with regulating PCa proliferation [38, 39]. Kim et al., also identified 10 AR-associated master-regulators (at a tissue level) associated with PCa in Black subjects. Four were differentially expressed in blood; all were decreased in peripheral blood of Black RSA patients (Figure 1E, Supplementary Table 1).
Singh et al., [21] have previously defined a series of genes linked to racial differences in PCa. We examined these genes identifying upregulation of CYP3A5 (FC: +1.78), genes involved in HSD17B (FC: +1.34 to +1.93) and a decreased in BCL-apoptosis genes (FC: -1.49 to - 1.91) (Supplementary Figure 1 and Supplementary Table 2).
Differential Gene Expression Analysis in Blood Samples from RSA and USA PCa Patients. 1A. Hierarchal clustering (heatmap) of gene expression profiles in blood samples from RSA-PCa vs USA-PCa. The heatmap was generated on Partek Flow® software, version 11.0.23.0918 (www.partek.com). 1B. Circos plots to visualize gene expression pattern in chromosomes between RSA and USA samples. Hotspots were identified in Chromosomes 1, 2, and 17. 1C. Comparative visualization of pathways between RSA-PCa and USA-PCa groups highlights oxidative phosphorylation and disease-related pathways. 1D. Peripheral blood expression of ethnic-associated tissue DEGs. The Immunoglobulin Kappa Variable 2D gene 29 was upregulated in blood, while both SH3KBP1 and STEAP4 were downregulated. These changes are consistent with tissue-based evaluations [20]. 1E. Four of ten previously identified master regulator transcription factors identified in PCa tumor tissues of Black men were downregulated in peripheral blood including CTCF, EP300, NR3C1 and SMARCA4.
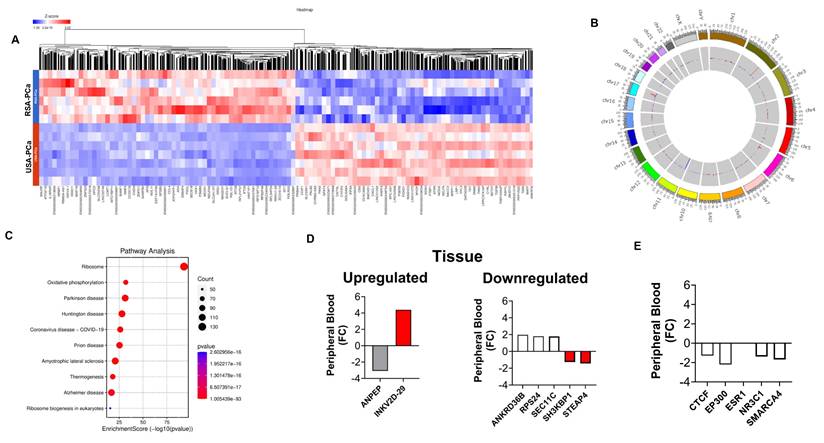
Differential gene expression analysis in blood samples from RSA and USA controls. 2A. Hierarchal clustering (heatmap) of gene expression profiles in blood samples from RSA-controls compared to USA controls. 2B. Circos plots to visualize gene expression pattern in chromosomes between RSA and USA samples. Hotspots were noted in Chromosomes 1, 2, 5, 14, 17, and 19. 2C. Comparative visualization of pathways between RSA-controls and USA-control groups highlights Protein Binding (Enzyme Regulatory Activity, mRNA Binding, and Zinc Ion Binding), Cell Division, and Cellular Responses to Insulin Stimulus. 2D. Peripheral blood expression of ethnic-associated tissue DEGs. Changes in controls were similar to those in PCa blood samples (see Figure 1D). 2E. The same master transcription factors as identified in PCa blood were downregulated in Black controls but ESR1 was additionally identified to be decreased in expression.
Interrogation of the PCa receptor cistrome [22] has identified that androgen signaling may contribute to changes in lipid metabolism and the immune response including cytokine signaling. Androgen receptor expression is known to be differentially expressed (between Caucasians and Blacks) [20, 40]. We evaluated families of genes for each of these pathways to evaluate whether the differences could be observed in peripheral blood (Supplementary Table 3). An evaluation of genes involved in lipid metabolism identified decreased expression of the majority of genes in Black PCa subjects but elevated expression of FABP5 (a known PCa marker [41]; FC: +2.15) was identified. LPL was also upregulated (FC: +2.24) in Black men. The majority of immune response genes had increased expression in Caucasian blood samples except for the CCL genes which was increased in Black men (FC: +1.43 to +3.68). COX2 was downregulated in Black men (FC: -2.44).
Differences between controls
We next compared gene expression in individuals in the control groups from RSA and the USA. Our analysis found 7,545 genes, with FC > 2 (p < 0.05) (Figure 2A). The five most significantly upregulated genes in RSA-Healthy controls were: IGHG1 (FC: 10.3), IGKV3-15 (FC: 8.6), IFI27 (FC: 8.5), MTCO1P40 (FC: 6.8) and RMRP (FC: 6.6) and the five most downregulated genes included GVINP1 (FC: -574.1), LY75-CD302 (FC: -313.4), ENSG00000263244 (FC: -109.8; a novel transcript, 3' overlapping Chromosome 16 ORF72, associated with obesity and cholesterol metabolism [also downregulated in RSA-PCa vs. USA-PCa]), ENSG00000272410 (FC: -20.0; a novel, uncharacterized protein associated with BMI) and HTATSF1P2 (FC: -17.0).
Figure 2B informs differences at the “healthy” control level and identify those differences that occur at baseline (in the absence of disease) with hotspots noted in Chromosome 1, 2, 5, 14, 17, and 19. These differences may reflect genetic, environmental or lifestyle differences that are associated with the RSA and USA populations.
Pathway analysis (Figure 2C) identified the following GO molecular functions including: Protein Binding (Enzyme Regulatory Activity, mRNA Binding, and Zinc Ion Binding). Cell Division, Microtubule Nucleation, Cell Polarity and Cellular Response to Insulin Stimulus were also identified (Supplementary Figure 2).
We next examined whether the tumor-associated gene expression differences [20], racial disparity genes [21] and AR-signaling gene differences [22] were also evident in controls. Levels and differences were similar as noted for PCa blood (see Figure 2D and 2E, and Supplementary Tables 1-3). The AR-master regulator associated transcription factor ESR1 was decreased in Black men (FC: -1.85). In the immune profile, IL15 and IL23 were upregulated (FC: +1.34 to +1.98).
Ethnic differences (PCa versus controls)
Given the differences between cancers (RSA vs. USA) and between controls from the two countries, our next approach was to compare and evaluate DEGs between cancers and control blood samples from the same country. A total of 16,533 transcripts were commonly detected (Supplementary Figure 3A). After statistical filtering (FC > 2, p < 0.05), we identified that ~10% of genes were commonly expressed (Supplementary Figure 3B).
RSA-PCa vs Controls: We identified 1,627 genes that were differentially expressed in PCa and control subjects with statistical significance (p-value < 0.05) and a fold change (FC) of > 2 (Supplementary Figure 4A-D). The five most differentially expressed genes include: ENSG00000270149 (FC: 7.2; a novel protein-coding transcript: TSTD1-F11R read-through linked to cholesterol metabolism), HBZ (FC: 5.9), ENSG00000288894 (FC: 5.6; a novel protein-coding transcript linked to cardiac and lipid metabolism), CHIT1 (FC: 5.05), ENSG00000263798 (FC: 4.9; a lnc RNA associated with cholesterol metabolism). The five most down regulated genes in RSA-PCa blood included ENSG00000287510 (FC: -10.2; lncRNA antisense to the LAPTM5 gene and related to immune function), AGBL3 (FC:-4.16), ENSG00000228748 (FC:-4.1; a lncRNA, associated with cardiovascular function), ENSG00000290680 (FC:-3.8; linked to BMI and obesity) and LINC02470 (FC:-3.8; a lncRNA that regulates bladder tumorigenicity). Circos identified hotspots in Chromosome 1, 15, 16 and 17.
The significant top five pathways were identified as ribosome biogenesis in eukaryotes, mitophagy, spliceosome, ribosomal related genes and hematologic cell lineages. The results indicate an increased activity or sensitivity of hematological cells in RSA-PCa. This could be a sign of an immune activity or modulation or other immune responses that may be associated with tumor biology in this ethnic group.
USA-PCa vs Controls: In this cohort, we identified 2,193 genes with significantly different expression when compared to controls groups, with a FC > 2 and a p-value < 0.05 (Supplementary Figure 5A-D). Of the genes that were upregulated in USA-PCa, the five most differentially expressed were: G0S2 (FC: 10.4), EGR1 (FC: 8.4), ENSG00000248993 (FC: 6.8; a novel MHC Class II Beta Chain N-Terminal Domain-Containing Protein associated with obesity), ENSG00000279753 (FC: 5.8; an uncharacterized gene associated with cholesterol metabolism) and OSM (FC: 4.4). The five most down regulated genes were: ENSG00000287510 (FC: -59.5; lncRNA antisense to the LAPTM5 gene and related to immune function), C4BPA (FC: -12.7), MTND4P12 (FC: -6.4), ENSG00000280205 (FC: -6.0; an uncharacterized gene related to height) and AIRN (FC: -5.5). Circos identified hotspots in Chromosomes 1, 5, 6, 15 and 19.
Pathway analysis identified three major pathways (protein binding, microRNA binding, and ribonucleoprotein complex binding). When the focus was on biological processes, four major areas were discerned, these included cellular localization, cell cycle, cellular response to stress and apoptosis. These pathways differ from those in the RSA samples (RSA-PCa vs. RSA-controls) identifying some intriguing ethnic differences in blood-derived cell activity in PCa.
Immune cell landscape analysis in USA and RSA populations
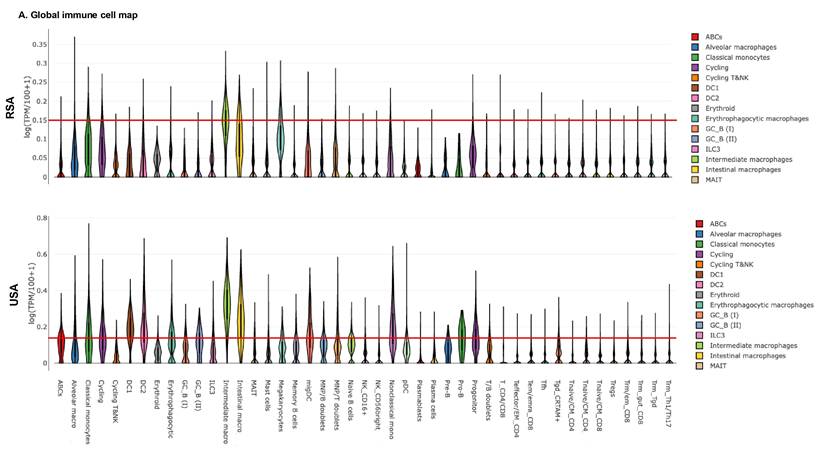
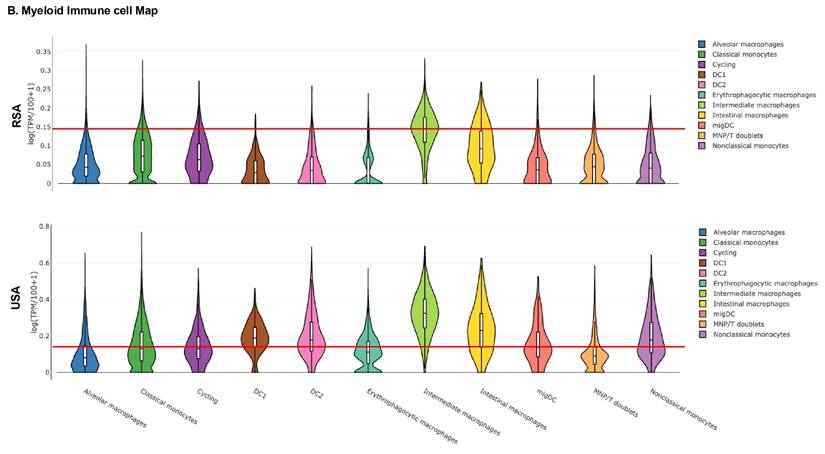
Given these findings, we next examined the peripheral blood immune landscape to better understand the population-based differences in the immune cell gene expression. For this, we performed global and myeloid immune cell analysis in RSA and USA cohorts based on DEGs. Figure 3A-B demonstrates global immune cell identification and variations in the PCa patients and the controls from RSA and the USA. We observed prominent differences in global immune cell landscape between these two populations. In general, the majority of the cell lineages were higher expressed in USA population (especially, ABCs, DC1, DC2, Erythrophagocytic Macrophages, Memory B cells and Naive B cells) compared to RSA (Figure 3A-B). The myeloid immune cell landscape using Myeloid Immune Cell mapping confirmed these differences (Macrophages, monocytes and dendritic cell types, Figure 3C-D).
Table 2 includes a summary of the molecular pathway differences across RSA and USA Cohorts. These range from hematopoietic cell development to differences in lipid biosynthesis and inflammatory responses and immune cell populations.
Evaluation of PROSTest genes in RNA-seq and qPCR
Lastly, we evaluated the expression of the 27 PROSTest genes (Table 3), which serves as established biomarkers of PCa identified in earlier studies at Wren Laboratories, in whole blood samples [16]. This was undertaken to evaluate whether this tissue-derived gene signature was detectable in blood and whether there were any significant differences in expression of the marker genes.
Exploring global immune cell maps in RSA and USA populations. 3A. Immune cell map analysis in RSA-PCa vs RSA-Controls (top) and USA-PCa vs USA-Controls(bottom) based on gene expression. Gene expression associated with various cell types and their log TPM values are included. The red lines are set at 0.15 as the scales are different. This provides for a comparison between the two groups which identifies over-expression of specific cell types including dendritic cells, macrophages and B cell types in Caucasian PCa peripheral blood. 3B. Myeloid immune cell map (top: RSA-PCa vs controls; bottom: USA-PCa vs. controls) of gene expression. The red lines are set at the same value (0.15) for ease of comparison. Dendritic cells, monocytes, and macrophage cell types are increased in Caucasian samples.
Summary of molecular pathway differences and biomarker validation across RSA and USA cohorts
| Category | RSA-PCa | USA-PCa | RSA-Controls | USA-Controls | Clinical/Translational Implications |
|---|---|---|---|---|---|
| Hematopoietic Lineages | ↑ Enrichment of hematopoietic cell pathways (systemic immune modulation) | - | - | - | Suggests tumor-driven immune reprogramming detectable in blood. |
| Immune Gene Expression | ↑ CCL genes ↑ IL7R, IL18RAP, IL32 | ↑ Multiple interleukins and NLRP genes | ↑ IL15, IL23A, IL32 | Similar but less pronounced as USA-PCa | Suggests interleukin gene expression differences between ethnic groups |
| Ribosome Biogenesis & RNA Splicing | ↑ Increased expression of ribosomal genes and splicing machinery | Similar but less pronounced | - | - | Onco-ribosomes and aberrant splicing promote tumor aggressiveness; blood-based detection may allow early stratification of high-risk patients. |
| ER Stress & Protein Export | ↑ Elevated ER stress and unfolded protein response (UPR) pathways | - | - | - | Links proteostasis stress to tumor growth and therapy resistance; potential therapeutic target. |
| Oxidative Phosphorylation (OXPHOS) | Distinctive differences vs. USA cohort | Distinctive differences vs. RSA cohort | - | - | Reflects population-specific mitochondrial metabolism; supports heterogeneity in cancer energetics and treatment response. |
| Baseline Molecular Signatures | - | - | ↑ Cell cycle, mRNA binding pathways | ↑ Insulin response pathways | Highlights genetic/environmental influences; critical for biomarker panel calibration across populations. |
| Fatty Acid Metabolism | Down-regulated compared to USA except for ↑ FABP5 and LPL | Upregulated | Similar but less pronounced as RSA-PCa | Similar but less pronounced as USA-PCa | Metabolic differences between ethnic groups |
| Testosterone and Estrogen regulation | ↑ CYP3A5 and HDS17B family of genes | - | ↑ HDS17B family of genes | - | Ethnic differences in Androgen regulation |
| PROSTest Validation | 26-gene panel validated; consistent RNAseq-qPCR correlation | 26-gene panel validated; consistent RNAseq-qPCR correlation | - | - | Robust across populations, but necessitates broader validation to ensure global utility. |
| Geographic & Socio-Economic Factors | Greater influence of genetic ancestry, environment, and infection exposure | Greater influence of lifestyle (diet, obesity, insulin signaling) and healthcare access | - | - | Socio-economic and geographic context strongly modulate molecular signatures; must be integrated into biomarker development strategies. |
Identification of PROSTest gene signature in RNA-seq data and comparison of qPCR ratios in RSA and USA clinical samples. RNA-seq data identified 26 of 27 PROSTest genes and validated these genes via qPCR on same clinical samples that were sequenced. We observed all 26 genes via qPCR and bar graphs were prepared based on ratios in USA and RSA samples.
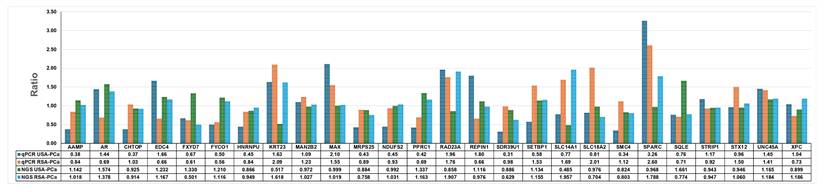
PROSTest genes identified in RNA-seq data in USA and RSA patients.
| RNA Sequencing | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| USA-PCa vs USA-Controls | RSA-PCa vs RSA-Controls | USA-PCa vs RSA-PCa | USA-Controls vs RSA-Controls | |||||||||
| Gene name | P-value | FDR | Ratio | P-value | FDR | Ratio | P-value | FDR | Ratio | P-value | FDR | Ratio |
| AAMP | 0.161 | 0.608 | 1.142 | 0.876 | 0.983 | 1.018 | 0.336 | 0.499 | 1.078 | 0.763 | 0.847 | 0.966 |
| AR | 0.110 | 0.533 | 1.574 | 0.376 | 0.843 | 1.378 | 0.725 | 0.825 | 1.128 | 0.981 | 0.990 | 0.993 |
| CHTOP | 0.334 | 0.755 | 0.925 | 0.262 | 0.789 | 0.914 | 0.186 | 0.328 | 0.935 | 0.439 | 0.583 | 0.928 |
| EDC4 | 0.010 | 0.213 | 1.232 | 0.265 | 0.791 | 1.167 | 0.000 | 0.000 | 1.497 | 0.002 | 0.009 | 1.425 |
| FXYD7 | 0.241 | 0.689 | 1.330 | 0.087 | 0.613 | 0.501 | 0.313 | 0.474 | 1.398 | 0.024 | 0.066 | 0.523 |
| FYCO1 | 0.013 | 0.244 | 1.210 | 0.419 | 0.869 | 1.116 | 0.000 | 0.000 | 1.432 | 0.015 | 0.044 | 1.329 |
| HNRNPU | 0.035 | 0.354 | 0.866 | 0.530 | 0.908 | 0.949 | 0.000 | 0.001 | 1.187 | 0.002 | 0.007 | 1.308 |
| KRT23 | 0.008 | 0.202 | 0.517 | 0.033 | 0.471 | 1.618 | 0.033 | 0.088 | 0.663 | 0.008 | 0.027 | 2.098 |
| MAN2B2 | 0.779 | 0.946 | 0.972 | 0.858 | 0.981 | 1.027 | 0.000 | 0.000 | 1.709 | 0.000 | 0.000 | 1.819 |
| MAX | 0.990 | 0.997 | 0.999 | 0.869 | 0.982 | 1.019 | 0.889 | 0.933 | 1.015 | 0.711 | 0.812 | 1.041 |
| MRPS25 | 0.257 | 0.702 | 0.884 | 0.006 | 0.305 | 0.758 | 0.005 | 0.019 | 1.338 | 0.202 | 0.334 | 1.154 |
| NDUFS2 | 0.892 | 0.975 | 0.992 | 0.699 | 0.953 | 1.031 | 0.717 | 0.820 | 1.024 | 0.343 | 0.491 | 1.072 |
| PPRC1 | 0.028 | 0.325 | 1.337 | 0.541 | 0.911 | 1.163 | 0.003 | 0.013 | 1.747 | 0.018 | 0.051 | 1.530 |
| RAD23A | 0.409 | 0.801 | 0.858 | 0.012 | 0.379 | 1.907 | 0.055 | 0.132 | 0.631 | 0.089 | 0.181 | 1.415 |
| REPIN1 | 0.276 | 0.716 | 1.116 | 0.867 | 0.982 | 0.976 | 0.001 | 0.007 | 1.399 | 0.112 | 0.216 | 1.232 |
| SDR39U1 | 0.390 | 0.787 | 0.886 | 0.013 | 0.385 | 0.629 | 0.397 | 0.560 | 0.874 | 0.004 | 0.015 | 0.623 |
| SETBP1 | 0.343 | 0.760 | 1.134 | 0.433 | 0.876 | 1.155 | 0.274 | 0.432 | 1.169 | 0.274 | 0.417 | 1.192 |
| SLC14A1 | 0.084 | 0.491 | 0.485 | 0.060 | 0.558 | 1.957 | 0.050 | 0.123 | 0.616 | 0.050 | 0.116 | 2.497 |
| SLC18A2 | 0.912 | 0.979 | 0.976 | 0.498 | 0.898 | 0.704 | 0.204 | 0.350 | 1.605 | 0.647 | 0.764 | 1.164 |
| SMC4 | 0.102 | 0.522 | 0.824 | 0.083 | 0.606 | 0.803 | 0.000 | 0.001 | 0.630 | 0.000 | 0.001 | 0.618 |
| SPARC | 0.892 | 0.975 | 0.968 | 0.024 | 0.443 | 1.788 | 0.056 | 0.134 | 0.612 | 0.606 | 0.731 | 1.135 |
| SQLE | 0.000 | 0.004 | 1.661 | 0.145 | 0.693 | 0.774 | 0.000 | 0.002 | 1.507 | 0.023 | 0.062 | 0.705 |
| STRIP1 | 0.380 | 0.780 | 0.943 | 0.560 | 0.917 | 0.947 | 0.074 | 0.165 | 1.147 | 0.055 | 0.124 | 1.160 |
| STX12 | 0.471 | 0.835 | 0.946 | 0.551 | 0.914 | 1.060 | 0.863 | 0.916 | 1.012 | 0.148 | 0.267 | 1.140 |
| UNC45A | 0.045 | 0.390 | 1.165 | 0.212 | 0.753 | 1.184 | 0.977 | 0.987 | 1.003 | 0.760 | 0.845 | 1.028 |
| XPC | 0.288 | 0.722 | 0.899 | 0.131 | 0.677 | 1.186 | 0.001 | 0.003 | 1.407 | 0.000 | 0.000 | 1.869 |
We employed two complementary methods, qPCR and RNA-seq. One of the 27 marker genes, HPN (hepsin), was not detected in the RNA-seq data and was excluded from analysis. Both qPCR and RNA-seq gave similar results for 26 PROSTest genes in the clinical samples (Pearson correlation: r=0.44, p=0.0012, n=30 pairs). This identified that these biomarkers are well and reproducibly detected irrespective of the method used (Figure 4, Table 3). The 26 PROSTest gene signature, was highly detectable in both populations with 100% sensitivity for differentiating PCa from healthy subjects (PROSTest genes and their RNA quantifications are listed in Table 3).
Discussion
The research pilot study examined gene expression patterns in peripheral whole blood samples collected from PCa patients and healthy donors from South Africa and the United States. The cohorts were chosen to meet appropriate standards for RNAseq studies (a minimum of 6 samples/cohort [23]), had similar ages (median ~60 years), were not being treated (either newly diagnosed or in active surveillance) and had similar Gleason grades and stages. This allowed us to minimize potential confounders and focus on evaluating differences in biology related to ethnicity and the environment.
RNA-seq data in these patients with localized, non-advanced tumors identified differential molecular variations between the two populations in terms of global gene expression patterns, pathway enrichment analysis and especially cell types involved in immune responses. These findings highlight some of the differences that genetic, environmental and socio-economic factors may have on PCa biology. They also suggest population-specific mechanisms that may be related or associated with disease (summarized in Table 3). We noted that PROSTest gene expression was similar between US and RSA PCa.
Authors have evaluated tumor samples for ethnic-associated gene and molecular pathway differences between Caucasia and Black PCa populations [20-22]. Black men show increased activity of inflammation, steroid hormone responses, and cancer progression-related pathways. Ethnic-based androgen and androgen receptor (AR) pathway differences are known to occur. We evaluated differences at a global level and then focused on some of the previously identified genes and pathways known to differ.
At a global level, increased expression of genes in blood that were involved in biosynthesis of ribosomes, splicing of RNA, and hematopoietic cell lineages in the RSA cohort compared to US patients was identified. These pathways have been extensively studied in solid tumors. Alterations in ribosomal biogenesis and splicing (at a tissue level) are known to be correlated with tumor development including aggressiveness and metastasis [42, 43]. PCa blood samples showed enhanced activity of pathways involved endoplasmic reticulum protein (ER) export stress which may be a key player in supporting tumor growth and treatment resistance [44]. The oxidative phosphorylation and disease-specific pathways were also distinguished as different between the RSA and USA groups. These findings accord with research work showing geographical and genetic heterogeneity in the function of the mitochondria and its relationship to cancer metabolism [45, 46]. An ER stress response leads to tumor cell activation which produces exosomes that contain UPR proteins which modify macrophage function and suppress T-cell activity [47]. Our data demonstrates that elevated ER stress/export pathways serve as biomarkers which reveal proteostatic stress caused by tumors while linking protein folding needs to cancer progression levels.
We also observed striking differences in the baseline gene expressions between the RSA-Controls and USA-Controls further highlighting the importance of population variation. RSA controls demonstrated over-representation of cell cycle and mRNA binding pathways whereas USA controls showed enrichment in insulin response signaling. Such divergence in baseline expression indicates the differences in the basic molecular activity which could be due to different genetic background and environmental factors of the two populations. For example, populations with higher prevalence of metabolic syndrome or obesity (USA) may demonstrate enhanced insulin signaling, while subjects of African genetic heritage (RSA) may preferentially express proliferative and translational machinery activation [48-50]. These baseline variations are similar to previously published large scale studies demonstrating significant ethnic variability in transcriptomes [51-54].
When we focused on specific genes and pathway differences noted at a tumor tissue level [20-22], we identified some intriguing overlaps between tissue-based expression and what was detectable in peripheral blood. Our study identified several tissue-associated differences were recapitulated in peripheral blood. For example, FABP5 was over-expressed in RSA-PCa blood. This co-ordinates lipid signaling to promote PCa metastasis [55]. Lipoprotein Lipase (LPL) is also linked to PCa by facilitating the uptake of fatty acids, which fuel tumor growth and proliferation [56]. This gene similarly was upregulated in RSA blood compared to Caucasian blood. The NF-κB pathway was also upregulated in Black subjects; this controls progression of PCa to androgen-independent growth [57]. Several genes transcribing interleukins were similarly upregulated in RSA-PCa samples. IL32 has a complex role in PCas, acting as a pro-tumor factor in metastatic disease by recruiting macrophages that promote tumor growth and metastasis through the CTSZ-TRA2A/IL-32/ITGA5 axis [58]. The IL7R (Interleukin-7 Receptor) was also upregulated and is implicated in PCa progression and potential therapeutic resistance [59]. Chemokine signaling plays a significant role in PCa progression, metastasis, and the tumor microenvironment by recruiting immune cells and promoting tumor cell survival and growth. CCL4 signaling promotes tumorigenesis, invasion, and recurrence by recruiting macrophages and influencing the tumor microenvironment [60], while CCL5 promotes tumor growth, metastasis, angiogenesis (new blood vessel formation), drug resistance, and the self-renewal of cancer stem cells through its receptor CCR5 [60]. Both genes were upregulated in Black subjects the expression and role of Suppressor of Cytokine Signaling (SOCS) proteins are complex and can be tumor-promoting or tumor-suppressive depending on the specific SOCS protein and cellular context [61]. Genes encoding proteins in this signaling pathway were upregulated too. Signals produced by tumors may affect blood cell development through mechanisms that lead to increased myeloid-derived suppressor cell (MDSC) numbers and altered lymphoid cell distribution [62, 63]. The process of immunologic reprogramming is a key factor that drives cancer development and determines treatment outcomes. The immune-associated differences in peripheral blood suggest some fundamental differences in hematopoietic pathways between Caucasian and Black subjects; this may potentially impact on PCa development and progression.
PROSTest Biomarker Validation: The PROSTest biomarker panel containing 26 genes showed consistent results between RSA and USA cohorts identifying that these genes are reliable and stable biomarkers across these two populations. Our results also reveal the necessity of global population validation since molecular differences do occur in blood-based markers. Previous studies have highlighted the issues in the biomarker validation across the various populations with an emphasis on the impact of genetics, environment and socio-economic factors on biomarker performance [64-67]. qPCR is a sensitive method that allows for the quantification of specific target genes, while RNA-seq offers the ability to confirm the presence and quantify the levels of expression of these genes in a global transcriptome setting [68].
Geographical and Socio-Economic Considerations: The differences in molecular signatures and the immune response DEGs (which likely translates to immune sub-population differences) between the RSA and USA cohorts are probably due to a genetically determined response to environmental exposures modulated by socio-economic factors. The fact that PCa has been noted to have different presentations in different geographical locations and ethnic groups has been well-established [69]. This has been shown in the androgen receptor signaling, metabolic pathways and the immune response of the cancer varies between populations [70-75]. Other factors that include diet (obesity, cholesterol metabolism, cardiovascular disease), infections and exposure to infectious agents and access to health care may also play a role in modulating gene expression and immunodynamics [76-80].
Our pilot study does have some limitations. The sample sizes are relatively small and uneven although we have attempted to meet requirements for RNAseq studies. One additional limitation was recruiting diverse ethnic groups, including RSA Caucasian and USA Black patients, to participate in the pilot study. Large-scale studies could further identify differences between these two groups (USA vs. RSA) and diverse racial pools, thereby enhancing generalizability and broader applicability. There may be a potential confounding by age, comorbidities, clinical stage distribution or sample handling differences but we have attempted to minimize these as far as possible. While no functional experiments, e.g., interleukin measurements in blood, were undertaken to confirm transcriptomic observations, these are being undertaken as part of a separate study. The qPCR vs. RNAseq expression of PROSTest genes, however, is a strength, that identifies the validity of the approach we used.
Conclusion
The present pilot study highlights the need to perform population-specific analysis in any blood-based molecular research in order to develop biomarker and therapeutic strategies. This study also demonstrates the heterogeneity of immunological and cellular pathways among two different geographic (and ethnic) populations highlighting the need to consider these differences when evaluating PCa pathobiology. For instance, RSA-PCa samples were found to have characteristics suggestive of an impaired antigen presentation which may impact the effectiveness of the immune system in identifying and eradicating cancerous cells. Conversely, USA-PCa samples displayed an inflammatory immune signature suggesting an overall immune activation (monocyte, dendritic cells and macrophage DEGs). These suggest that blood samples from PCa are associated with different immune profiles, depending on the population. Such differences should be considered in studies as well as in clinical practice. In biomarker development, inclusion of subjects from different countries should be a principal focus in future genomic studies. Increasing the participation of different populations will help to increase the transferability of biomarkers and the interventions thus making them relevant to a wider population. Such inclusivity is especially important for the development of precision medicine which aims at providing individualized treatment based on the patient's genetic background as well as socio-economic environment.
Clinical Implications
The determination of population attributable gene expression and immune signatures is important in PCa diagnosis and management. The alterations suggest different immune:cancer relationships that may have an impact on presentation, development and treatment of PCa. The confirmation of the PROSTest genes in both ethnic populations identifies that this non-invasive liquid biopsy-based diagnostic is a viable and effective tool for PCa.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
Funding
This research was funded by the South African Medical Research Council through grant SHIP-RFA-01-2019 for precision medicine and pharmacogenomics.
Data availability
The RNA sequencing data from the USA and South Africa datasets analyzed in this study are available in the ArrayExpress gene expression repository under accession number [E-MTAB-14952]
Informed consent statement
All patient samples were collected with informed and written consent only.
Competing Interests
The authors have declared that no competing interest exists.
References
1. James ND, Tannock I, N'Dow J, Feng F, Gillessen S, Ali SA. et al. The Lancet Commission on prostate cancer: planning for the surge in cases. Lancet. 2024;403:1683-722
2. Siegel RL, Kratzer TB, Giaquinto AN, Sung H, Jemal A. Cancer statistics, 2025. CA Cancer J Clin. 2025;75:10-45
3. Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M. et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2017;71:618-29 doi: 10.1016/j.eururo.2016.08.003. Epub Aug 25
4. Bell KJ, Del Mar C, Wright G, Dickinson J, Glasziou P. Prevalence of incidental prostate cancer: A systematic review of autopsy studies. Int J Cancer. 2015;137:1749-57 doi: 10.002/ijc.29538. Epub 2015 Apr 21
5. Das S, Salami SS, Spratt DE, Kaffenberger SD, Jacobs MF, Morgan TM. Bringing Prostate Cancer Germline Genetics into Clinical Practice. J Urol. 2019;5:0000000000000137
6. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34 doi: 10.3322/caac.21551. Epub 2019 Jan 8
7. Marima R, Mbeje M, Hull R, Demetriou D, Mtshali N, Dlamini Z. Prostate cancer disparities and management in southern Africa: Insights into practices, norms and values. Cancer Management and Research. 2022:3567-79
8. El Khoury CJ. Application of Geographic Information Systems (GIS) in the Study of Prostate Cancer Disparities: A Systematic Review. Cancers. 2024;16:2715
9. Halim A. Biomarkers, Diagnostics and Precision Medicine in the Drug Industry: Critical Challenges, Limitations and Roadmaps for the Best Practices: Academic Press; 2019
10. Trujillo B, Wu A, Wetterskog D, Attard G. Blood-based liquid biopsies for prostate cancer: clinical opportunities and challenges. Br J Cancer. 2022;127:1394-402
11. Ladurner M, Wieser M, Eigentler A, Seewald M, Dobler G, Neuwirt H. et al. Validation of Cell-Free RNA and Circulating Tumor Cells for Molecular Marker Analysis in Metastatic Prostate Cancer. Biomedicines. 2021 9
12. Nilsson RJ, Balaj L, Hulleman E, van Rijn S, Pegtel DM, Walraven M. et al. Blood platelets contain tumor-derived RNA biomarkers. Blood. 2011;118:3680-3
13. Olmos D, Brewer D, Clark J, Danila DC, Parker C, Attard G. et al. Prognostic value of blood mRNA expression signatures in castration-resistant prostate cancer: a prospective, two-stage study. Lancet Oncol. 2012;13:1114-24 doi: 10.016/S470-2045(12)70372-8. Epub 2012 Oct 9
14. Ross RW, Galsky MD, Scher HI, Magidson J, Wassmann K, Lee GS. et al. A whole-blood RNA transcript-based prognostic model in men with castration-resistant prostate cancer: a prospective study. Lancet Oncol. 2012;13:1105-13 doi: 10.016/S470-2045(12)70263-2. Epub 2012 Oct 9
15. Danila DC, Anand A, Schultz N, Heller G, Wan M, Sung CC. et al. Analytic and clinical validation of a prostate cancer-enhanced messenger RNA detection assay in whole blood as a prognostic biomarker for survival. European urology. 2014;65:1191-7
16. Modlin IM, Kidd M, Drozdov IA, Boegemann M, Bodei L, Kunikowska J. et al. Development of a multigenomic liquid biopsy (PROSTest) for prostate cancer in whole blood. Prostate. 2024;84:850-65
17. Rosin RD, Haynes A, Kidd M, Drozdov I, Modlin I, Halim A. Evaluation of a multigenomic liquid biopsy (PROSTest) for prostate cancer detection and follow-up in a Caribbean population. Cancer epidemiology. 2024;92:102642
18. Janivara R, Chen WC, Hazra U, Baichoo S, Agalliu I, Kachambwa P. et al. Heterogeneous genetic architectures of prostate cancer susceptibility in sub-Saharan Africa. Nat Genet. 2024;56:2093-103
19. Wang A, Shen J, Rodriguez AA, Saunders EJ, Chen F, Janivara R. et al. Characterizing prostate cancer risk through multi-ancestry genome-wide discovery of 187 novel risk variants. Nat Genet. 2023;55:2065-74
20. Kim M, Tamukong P, Galvan GC, Yang Q, De Hoedt A, Freeman MR. et al. Prostate cancers with distinct transcriptional programs in Black and White men. Genome Med. 2024;16:92
21. Singh SK, Lillard JW Jr, Singh R. Molecular basis for prostate cancer racial disparities. Front Biosci (Landmark Ed). 2017;22:428-50
22. Berchuck JE, Adib E, Abou Alaiwi S, Dash AK, Shin JN, Lowder D. et al. The Prostate Cancer Androgen Receptor Cistrome in African American Men Associates with Upregulation of Lipid Metabolism and Immune Response. Cancer Res. 2022;82:2848-59
23. Schurch NJ, Schofield P, Gierliński M, Cole C, Sherstnev A, Singh V. et al. How many biological replicates are needed in an RNA-seq experiment and which differential expression tool should you use? Rna. 2016;22:839-51
24. Kidd M, Drozdov IA, Matar S, Gurunlian N, Ferranti NJ, Malczewska A. et al. Utility of a ready-to-use PCR system for neuroendocrine tumor diagnosis. PloS one. 2019;14:e0218592
25. Kidd M, Nadler B, Mane S, Eick G, Malfertheiner M, Champaneria M. et al. GeneChip, geNorm, and gastrointestinal tumors: novel reference genes for real-time PCR. Physiological genomics. 2007;30:363-70
26. Modlin IM, Kidd M, Drozdov IA, Boegemann M, Bodei L, Kunikowska J. et al. Development of a multigenomic liquid biopsy (PROSTest) for prostate cancer in whole blood. The Prostate. 2024;84:850-65
27. Tang D, Chen M, Huang X, Zhang G, Zeng L, Zhang G. et al. SRplot: A free online platform for data visualization and graphing. PloS one. 2023;18:e0294236
28. Koduru SV, Leberfinger AN, Ozbolat IT, Ravnic DJ. Navigating the Genomic Landscape of Human Adipose Stem Cell-Derived β-Cells. Stem Cells and Development. 2021;30:1153-70
29. Koduru SV, Tiwari AK, Leberfinger A, Hazard SW, Kawasawa YI, Mahajan M. et al. A comprehensive NGS data analysis of differentially regulated miRNAs, piRNAs, lncRNAs and sn/snoRNAs in triple negative breast cancer. Journal of Cancer. 2017;8:578
30. Luo W, Brouwer C. Pathview: an R/Bioconductor package for pathway-based data integration and visualization. Bioinformatics. 2013;29:1830-1
31. Yu G, Wang L-G, Han Y, He Q-Y. clusterProfiler: an R Package for Comparing Biological Themes Among Gene Clusters. OMICS: A Journal of Integrative Biology. 2012;16:284-7
32. Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q. et al. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020;48:W509-w14
33. Li T, Fan J, Wang B, Traugh N, Chen Q, Liu JS. et al. TIMER: A Web Server for Comprehensive Analysis of Tumor-Infiltrating Immune Cells. Cancer research. 2017;77:e108-e10
34. Li B, Severson E, Pignon JC, Zhao H, Li T, Novak J. et al. Comprehensive analyses of tumor immunity: implications for cancer immunotherapy. Genome Biol. 2016;17:174
35. Sturm G, Finotello F, Petitprez F, Zhang JD, Baumbach J, Fridman WH. et al. Comprehensive evaluation of transcriptome-based cell-type quantification methods for immuno-oncology. Bioinformatics. 2019;35:i436-i45
36. Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D. et al. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639-45
37. Conti DV, Darst BF, Moss LC, Saunders EJ, Sheng X, Chou A. et al. Trans-ancestry genome-wide association meta-analysis of prostate cancer identifies new susceptibility loci and informs genetic risk prediction. Nat Genet. 2021;53:65-75
38. Fan S, Xia Z, Liu W, Zhu Y, Liu X, Gu P. et al. STEAP4 facilitates growth, migration, and invasion of prostate carcinoma through upregulation of NOTCH4. Faseb j. 2025;39:e70508
39. Mukhopadhyay C, Yang C, Xu L, Liu D, Wang Y, Huang D. et al. G3BP1 inhibits Cul3(SPOP) to amplify AR signaling and promote prostate cancer. Nat Commun. 2021;12:6662
40. Lowder D, Rizwan K, McColl C, Paparella A, Ittmann M, Mitsiades N. et al. Racial disparities in prostate cancer: A complex interplay between socioeconomic inequities and genomics. Cancer Lett. 2022;531:71-82
41. O'Sullivan SE, Kaczocha M. FABP5 as a novel molecular target in prostate cancer. Drug Discov Today. 2020
42. Sulima SO, Hofman IJ, De Keersmaecker K, Dinman JD. How ribosomes translate cancer. Cancer discovery. 2017;7:1069-87
43. De Keersmaecker K, Sulima SO, Dinman JD. Ribosomopathies and the paradox of cellular hypo- to hyperproliferation. Blood. 2015;125:1377-82
44. Wang M, Kaufman RJ. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature. 2016;529:326-35
45. Stewart JB, Chinnery PF. Extreme heterogeneity of human mitochondrial DNA from organelles to populations. Nature Reviews Genetics. 2021;22:106-18
46. McClellan J, King M-C. Genetic heterogeneity in human disease. Cell. 2010;141:210-7
47. Mahadevan NR, Rodvold J, Sepulveda H, Rossi S, Drew AF, Zanetti M. Transmission of endoplasmic reticulum stress and pro-inflammation from tumor cells to myeloid cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:6561-6
48. O'Connell GC, Wang J, Smothers C. Donor white blood cell differential is the single largest determinant of whole blood gene expression patterns. Genomics. 2023;115:110708
49. Adeyemo A, Rotimi C. Genetic variants associated with complex human diseases show wide variation across multiple populations. Public Health Genomics. 2010;13:72-9
50. Fletcher SA, Marchese M, Cole AP, Mahal BA, Friedlander DF, Krimphove M. et al. Geographic Distribution of Racial Differences in Prostate Cancer Mortality. JAMA Network Open. 2020;3:e201839-e
51. Harris BH, Barberis A, West CM, Buffa FM. Gene expression signatures as biomarkers of tumour hypoxia. Clinical Oncology. 2015;27:547-60
52. Gnjatic S, Bronte V, Brunet LR, Butler MO, Disis ML, Galon J. et al. Identifying baseline immune-related biomarkers to predict clinical outcome of immunotherapy. Journal for immunotherapy of cancer. 2017;5:1-18
53. Feng Z, Prentice R, Srivastava S. Research issues and strategies for genomic and proteomic biomarker discovery and validation: a statistical perspective. Pharmacogenomics. 2004;5:709-19
54. Eady JJ, Wortley GM, Wormstone YM, Hughes JC, Astley SB, Foxall RJ. et al. Variation in gene expression profiles of peripheral blood mononuclear cells from healthy volunteers. Physiological genomics. 2005;22:402-11
55. Carbonetti G, Wilpshaar T, Kroonen J, Studholme K, Converso C, d'Oelsnitz S. et al. FABP5 coordinates lipid signaling that promotes prostate cancer metastasis. Sci Rep. 2019;9:18944
56. Kuemmerle NB, Rysman E, Lombardo PS, Flanagan AJ, Lipe BC, Wells WA. et al. Lipoprotein lipase links dietary fat to solid tumor cell proliferation. Mol Cancer Ther. 2011;10:427-36
57. Jin RJ, Lho Y, Connelly L, Wang Y, Yu X, Saint Jean L. et al. The nuclear factor-kappaB pathway controls the progression of prostate cancer to androgen-independent growth. Cancer Res. 2008;68:6762-9
58. Chen S, Deng B, Liu Y, Xie L, Xu J, Hu R. et al. The CTSZ-TRA2A-IL32 axis defines a targetable macrophage-dependent pathway in metastatic prostate cancer. Journal of translational medicine. 2025;23:865
59. Wang C, Kong L, Kim S, Lee S, Oh S, Jo S. et al. The Role of IL-7 and IL-7R in Cancer Pathophysiology and Immunotherapy. International journal of molecular sciences. 2022 23
60. Izumi K, Mizokami A. Suppressive Role of Androgen/Androgen Receptor Signaling via Chemokines on Prostate Cancer Cells. Journal of clinical medicine. 2019 8
61. Jafarzadeh A, Zandvakili R, Jafarzadeh Z, Nemati M. Dysregulated expression of the suppressors of cytokine signaling (SOCS) contributes to the development of prostate cancer. Pathol Res Pract. 2024;262:155558
62. Veglia F, Sanseviero E, Gabrilovich DI. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat Rev Immunol. 2021;21:485-98
63. Kim J, Bae JS. Tumor-Associated Macrophages and Neutrophils in Tumor Microenvironment. Mediators Inflamm. 2016;2016:6058147
64. Guyer H, Ofstedal MB, Lessof C, Cox K. The benefits and challenges of collecting physical measures and biomarkers in cross-national studies. 2017.
65. Abrao Nemeir I, Saab J, Hleihel W, Errachid A, Jafferzic-Renault N, Zine N. The advent of salivary breast cancer biomarker detection using affinity sensors. Sensors. 2019;19:2373
66. López de Maturana E, Alonso L, Alarcón P, Martín-Antoniano IA, Pineda S, Piorno L. et al. Challenges in the integration of omics and non-omics data. Genes. 2019;10:238
67. Kataria S, Ravindran V. Harnessing of real-world data and real-world evidence using digital tools: utility and potential models in rheumatology practice. Rheumatology. 2022;61:502-13
68. Ståhlberg A, Bengtsson M. Single-cell gene expression profiling using reverse transcription quantitative real-time PCR. Methods. 2010;50:282-8
69. Rebbeck TR. Prostate Cancer Genetics: Variation by Race, Ethnicity, and Geography. Semin Radiat Oncol. 2017;27:3-10
70. Culig Z, Santer FR. Androgen receptor signaling in prostate cancer. Cancer and Metastasis Reviews. 2014;33:413-27
71. Aurilio G, Cimadamore A, Mazzucchelli R, Lopez-Beltran A, Verri E, Scarpelli M. et al. Androgen receptor signaling pathway in prostate cancer: from genetics to clinical applications. Cells. 2020;9:2653
72. Ben-Batalla I, Vargas-Delgado ME, Von Amsberg G, Janning M, Loges S. Influence of androgens on immunity to self and foreign: effects on immunity and cancer. Frontiers in immunology. 2020;11:1184
73. Dotto GP, Buckinx A, Özdemir BC, Simon C. Androgen receptor signalling in non-prostatic malignancies: challenges and opportunities. Nature Reviews Cancer. 2024:1-16
74. Yuan J, Kensler KH, Hu Z, Zhang Y, Zhang T, Jiang J. et al. Integrative comparison of the genomic and transcriptomic landscape between prostate cancer patients of predominantly African or European genetic ancestry. PLoS genetics. 2020;16:e1008641
75. Rebbeck TR. Cancer in sub-saharan africa. Science. 2020;367:27-8
76. Ramalho R, Rao M, Zhang C, Agrati C, Ippolito G, Wang F-S. et al. Immunometabolism: new insights and lessons from antigen-directed cellular immune responses. Seminars in immunopathology: Springer. 2020 p. 279-313
77. Engel DR, Wagenlehner FM, Shevchuk O. Scientific Advances in Understanding the Pathogenesis, Diagnosis, and Prevention of Urinary Tract Infection in the Past 10 Years. Infectious Disease Clinics. 2024
78. Cuevas A, Saavedra N, Salazar LA, Abdalla DS. Modulation of immune function by polyphenols: possible contribution of epigenetic factors. Nutrients. 2013;5:2314-32
79. Yang F, Yang Y, Zeng L, Chen Y, Zeng G. Nutrition metabolism and infections. Infectious Microbes & Diseases. 2021;3:134-41
80. Paul B, Barnes S, Demark-Wahnefried W, Morrow C, Salvador C, Skibola C. et al. Influences of diet and the gut microbiome on epigenetic modulation in cancer and other diseases. Clinical epigenetics. 2015;7:1-11
Author contact
![]() Corresponding author: Srinivas V Koduru, PhD, Director, Transcriptomics, Wren Laboratories LLC, Branford CT, USA, Email: skodurucom, Phone: +1 (475)221-8056.
Corresponding author: Srinivas V Koduru, PhD, Director, Transcriptomics, Wren Laboratories LLC, Branford CT, USA, Email: skodurucom, Phone: +1 (475)221-8056.

 Global reach, higher impact
Global reach, higher impact